Bromine trifluoride
Bromine ( III ) fluoride
Colorless to yellowish liquid
Liquid
8.77 ° C
125.72 ° C
1.4536 (25 ° C)
Template: Infobox chemical / molecular formula search is not possible
Bromine trifluoride is a colorless to yellowish liquid which chemically is a connection between the halogens bromine and fluorine. It was discovered in 1906 by Paul Lebeau.
Production and representation
Bromine trifluoride can be obtained by reaction of bromine with fluorine.
The presentation by disproportionation of bromine fluoride is possible.
Very pure bromine trifluoride can be obtained by direct fluorination of bromine with trichlorofluoromethane at -40 ° C.
Properties
Bromine trifluoride is a colorless, fuming liquid in the air. It is very reactive and attacks the skin greatly. In solid form, it exists as long prisms.
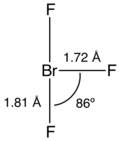
Similar to chlorine trifluoride and bromine trifluoride Iodtrifluorid is a T- shaped molecule. The distance between the axial bromine and fluorine is in each case 181 pm, while the distance to the center of fluorine is 172 pm. The angle between the center and the axial fluorine is 86.2 °.
Use
Bromine trifluoride is run a good solvent for the reactions under strongly oxidizing conditions. It is also a powerful fluorinating agents and can be used for the synthesis of the corresponding organic and inorganic compounds.