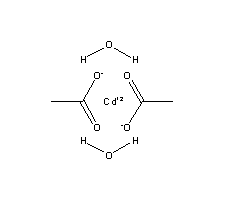
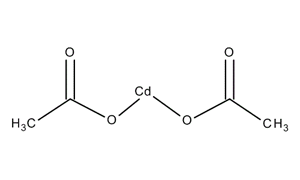
Cadmium acetate
Acetate of cadmium
- 543-90-8 (anhydrous)
- 5743-04-4 (dihydrate )
Colorless columnar crystals
Fixed
- 2.01 g · cm -3 ( dihydrate)
- 2.341 g · cm -3 ( anhydrous)
Soluble in water
Attention
333 mg · kg -1 ( LD50, rat, oral)
Template: Infobox chemical / molecular formula search available
Cadmium is a chemical compound of the cadmium and a salt of acetic acid. It is a white, hygroscopic substance with typical, weak essigartigem odor.
Production and representation
Cadmium acetate dihydrate precipitates from an aqueous acetic acid solution. Direct it can be recovered from cadmium nitrate and acetic anhydride.
Properties
At 130 ° C cadmium acetate hydrate are from the crystal water and the anhydrous cadmium acetate is formed. In water it dissolves with a neutral pH. Highly soluble, it is also in ethanol.
Use
Cadmium is used in the manufacture of porcelain for producing iridescent effects. Moreover, it is a reagent in analytical chemistry for the detection of sulfur, selenium and tellurium.