Carbonate
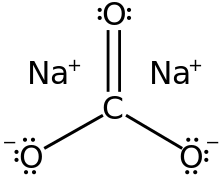
Carbonates ( technical terminology ) or carbonates are the salts and esters of carbonic acid ( H2CO3 ). From the dibasic ( dibasic ) acid, two series of salts derived from this, namely, the hydrogen carbonates, also known as primary carbonates having the general formula and the secondary MIHCO3 carbonates having the general formula MI2CO3. The secondary carbonates based on the doubly negatively charged carbonate ion CO32-.
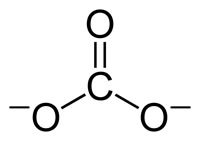
The esters of carbonic acid with the general formula: R1 -O- C (= O)-O -R2, wherein R1 and R2 are carbon-containing alkyl or aryl radicals are also known, and carbonates described in carbonic acid esters. Carbonate polymers have the general structural formula (O -R -O -C ( = O) -) n are discussed under polycarbonates.
From the non-existing in free form orthocarbonic (C (OH) 4 ) are derived from the ortho- acid ester having the structural formula C ( OR) 4.
Properties of carbonates
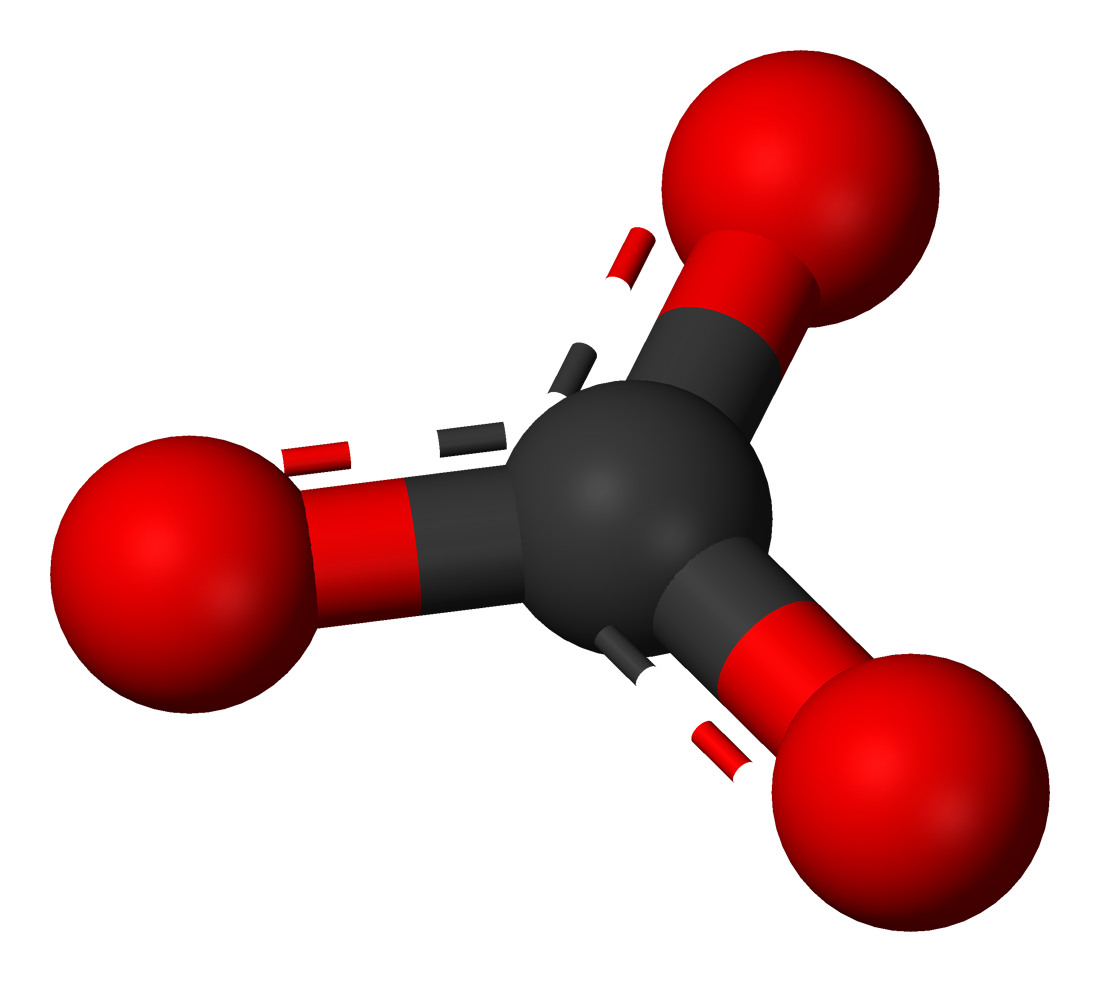
The carbonate anion is completely planar built, with 120 ° bond angles between the oxygen atoms. The distances of all three O atoms to the central carbon atom are the same and are about 130 pm between the length of CO single bonds (143 pm ) and C = O double bonds (123 pm ).
Carbonates are ionic salts and therefore at room temperature is generally crystalline solids. The carbonate anion brings no intrinsic color in the compounds, so that their color is optionally determined by the particular cation. Carbonates are odorless. With the exception of alkali metal carbonates, they are only slightly soluble in water, so that most of the metals are precipitated in the reaction with alkali metal carbonates.
Occurrence and key representatives
Carbonates are ubiquitous in nature, usually in the form of various minerals. In the scheme of minerals by Strunz ( 9th edition ) they form a common mineral class together with the related nitrates. In the 8th edition of the outdated Strunz'schen systematics as well as in the commonly used in the English-speaking classification of minerals according to Dana addition, the borates are not included with the class.
Important and well-known representatives of the carbonates include the barium Witherite; calcium carbonates aragonite, calcite and vaterite; the iron carbonate siderite ( siderite ); the basic copper carbonates azurite and malachite; the magnesite magnesium carbonate; manganese carbonate rhodochrosite ( rhodochrosite ); the sodium carbonates Gaylussite, Natrit, Pirssonit, soda and Trona, Smithsonite zinc carbonate ( calamine ) and Bastnäsit as a raw material for the extraction of rare earth metals. Other representatives of the Carbonatfamilie are dolomite ( calcium magnesium carbonate ) and the only known as a substance mixture in nature " potash " (potassium carbonate ). The total amount of carbon contained in the lithosphere is about 2.9 x 1016 t.
Furthermore, also includes the hydrosphere of the seas, lakes and rivers large amounts of dissolved carbonates and almost all living things carry both dissolved carbonates in them or use insoluble carbonates as scaffold material. Geologically occur mostly as carbonates sedimentary rocks (limestone) or even rare metamorphic igneous rocks ( carbonatite ) on.
Strontianite
Witherite
Azurite (blue) and malachite (green)
Rhodochrosite (red)
Violet to pink Smithsonite
Reactions of carbonates
- Reaction between carbon dioxide and carbonate:
This reaction takes place in the solution of limestone in carbonated groundwater from. It is the source of water hardness.
- Dissociation in water:
- Decomposition when heated:
This is the reaction in the production of lime.
See also: carbonate -silicate cycle
Alkaline reaction
The carbonate ions react with water to form hydrogen and hydroxide ions. It is an acid -base reaction.
Although this equilibrium is on the left, react by the increased concentration of hydroxide solutions of carbonates but distinctly alkaline.
Detection of carbonates
Carbonate ion ( CO32 - ) can be detected by the addition of hydrochloric acid, is formed in the carbon dioxide:
Carbon dioxide is introduced into the detection reagent lime or barium hydroxide. There it produces a white turbidity of calcium - or barium:
The quantitative detection of low carbonate concentrations in water samples is carried out together with the determination of hydrogen carbonates often by titration with hydrochloric acid ( " SBV " ), the material to be measured at the beginning of a pH above 8.3, so the hydrochloric acid consumption is taken to reach pH 8.3 equivalent of carbonate. The additional consumption of acid to reach a pH of 4.3 is the sum of carbonate and bicarbonate. In waters with pH values below 8.3 and titrate equal only to 4.3 ( only hydrogen ) and calculates the original, then very small proportion of carbonate ions from the dissociation of carbonic acid.
Carbonates and bicarbonates may also be determined by ion - HPLC or capillary electrophoresis. In both cases a " total carbonate " is identified and the components of carbonate, bicarbonate, and again calculated "free carbonic acid " in consideration of pH, ionic strength and temperature from the dissociation of carbonic acid.