Cellular respiration
As aerobic respiration ( cellular respiration, internal respiration ) are metabolic processes in cells of living organisms referred in which the expenses occasioned by various oxidative metabolic processes and linked to specific vectors hydrogen atoms are oxidized. This is molecular, elemental oxygen (O2) as the oxidizing agent, which is thereby reduced to water. The purpose of the aerobic respiration is to provide energy in the form of adenosine triphosphate (ATP). The term aerobic respiration is particularly used for the biochemical processes of the respiratory chain in the inner membrane of the mitochondria is synthesized at the end of ATP.
Other forms of breathing - in terms of gas exchange of living organisms - are subsumed under the concept of external respiration.
Survey
In the following the use of the energy from the oxidation of D-glucose (dextrose ) is represented by cells. Cells can obtain energy by oxidation of other substances, however, the oxidation of glucose is the most commonly used energy source.
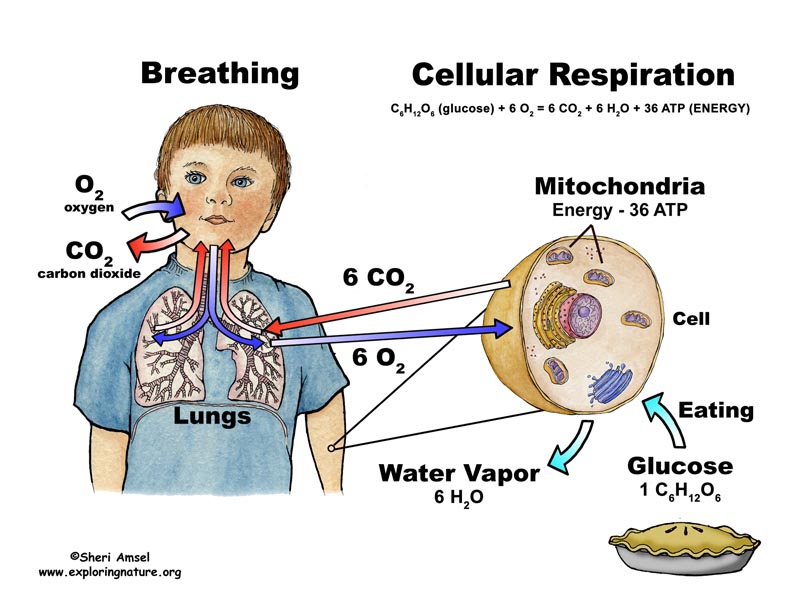
Cells take their energy supply on glucose. It is oxidized in the cytoplasm of eukaryotes and in the mitochondria completely to carbon dioxide and water. The total equation is:
The change in Gibbs energy under standard conditions is, however, at pH = 7 for this reaction ΔG0 '= -2880 kJ per mole of glucose. If the actual conditions of these standard conditions, so too is the amount of change in Gibbs another, he can deviate significantly from the standard value. In living systems, standard conditions are not usually given and often change during the material implementation. The amount of change in Gibbs energy under standard conditions in animals thus provides only an indication of the energy released during a chemical substance implementation.
Hydrogen separated from the decomposition products of the glucose molecules, and transported by means of hydrogen transfer agents ( nicotinamide adenine dinucleotide, NAD) to the mitochondria - This chemical material conversion are in a number of complex reaction steps - many redox reactions. There, the hydrogen atoms react in the respiratory chain with oxygen to form water (sometimes simply as "biological oxyhydrogen reaction " means ); the glucose molecules are ultimately completely oxidized. At the end of the degradation process, the cell gains with the help of liberated during the biological oxidation of hydrogen energy, the energy- rich compound adenosine triphosphate ( ATP). It serves as an energy carrier and short-term energy storage and is required for many metabolic processes as a universal energy source.
The summary equation of cellular respiration corresponds to read from right to left, the net reaction equation for oxygenic photosynthesis.
Operation of the process
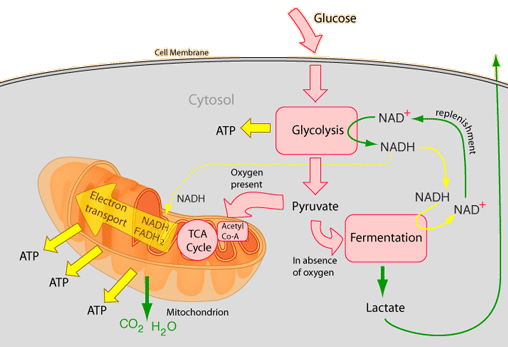
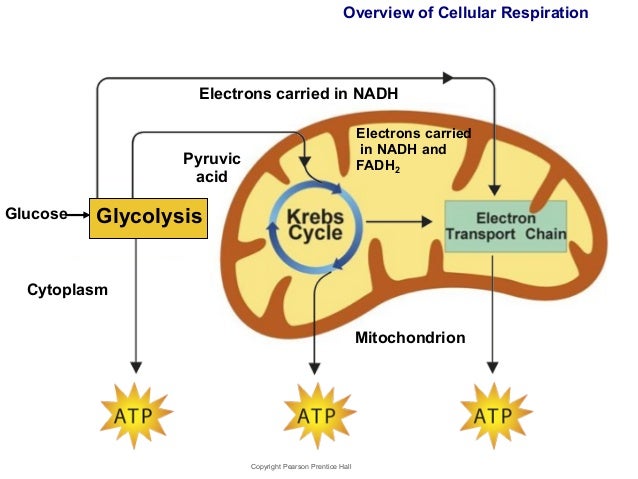
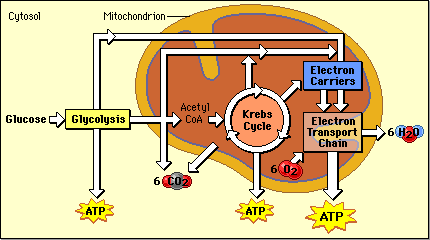
Cellular respiration is a process that will be implemented at the high-energy in low-energy materials. In the case of cellular respiration, the glucose molecule C6H12O6 in a long series of steps to C1 - bodies ( CO2) and water ( H2O) is mostly oxidized. This degradation can be divided into four sections:
The overall balance of cellular respiration can be formulated as follows:
Glycolysis
→ Main article: glycolysis
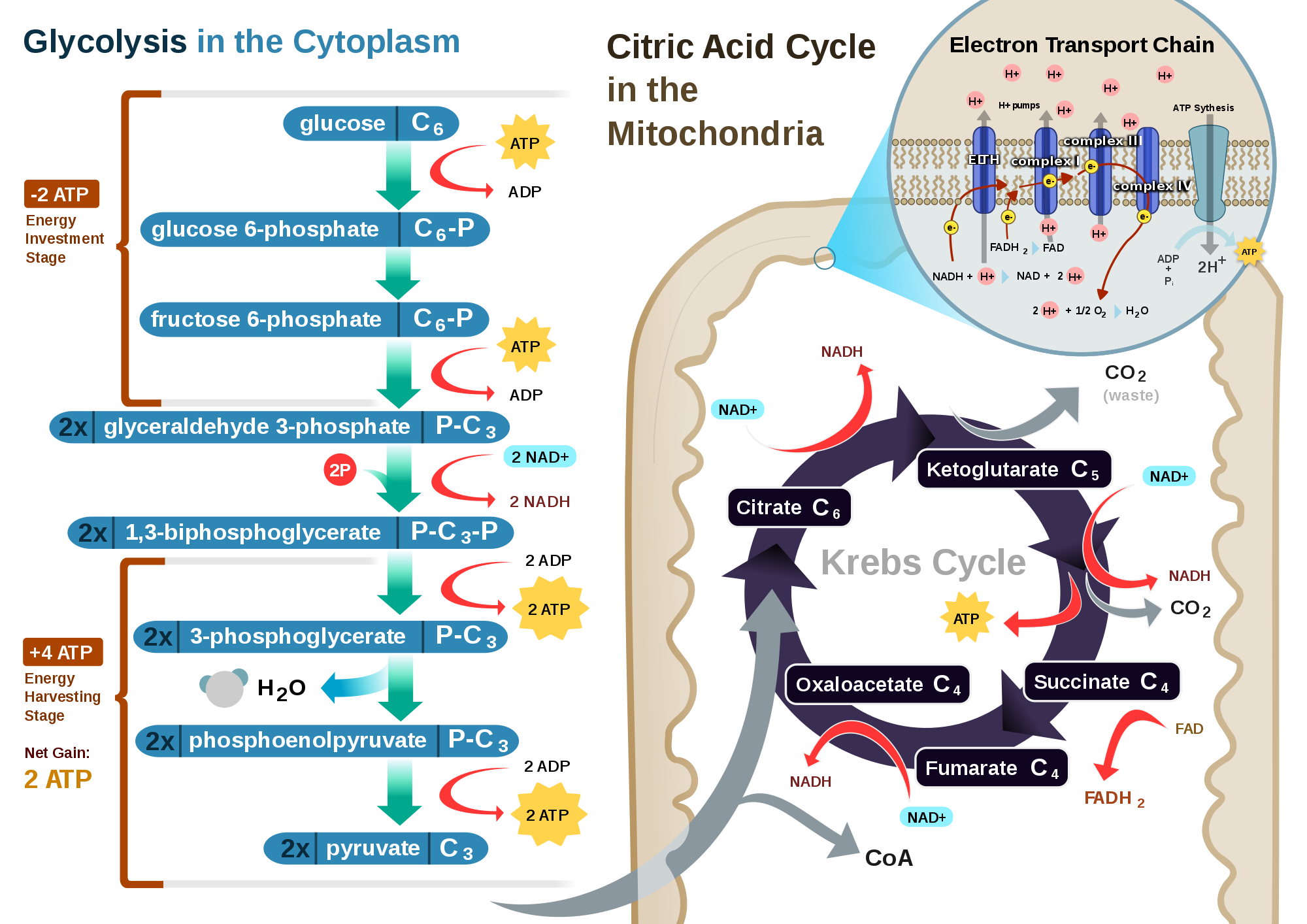
Glycolysis ( = sugar decomposition ) runs in the cytoplasm. In this operation, D-glucose is cleaved. This is done by dual phosphorylation so that glucose-6 -phosphate, fructose -6-phosphate produced and fructose-1 ,6 -bisphosphate. For these processes, two molecules of ATP to 2 ADP molecules are dephosphorylated. Phosphorylation of glucose is now in the activated state. This C6 - body is then split into two C3 - body, in a molecule dihydroxyacetone phosphate ( DHAP ) and one molecule of glyceraldehyde -3-phosphate (GAP ). Only the glyceraldehyde -3 -phosphate is further reduced, so the DHAP is isomerized to this.
Another molecule inorganic phosphate is deposited and GAP is oxidized to glyceric acid -1 ,3- biphosphate (1.3 bpg ) is created. The electrons are transferred to the hydrogen carrier NAD ( nicotinamide adenine dinucleotide ). In the further step, a phosphate group (P ) is transmitted to ADP, ATP and glyceric acid so that 3-phosphate (3- PG ) are formed. PG - 3 is isomerized to glyceric acid - 2-phosphate (2- PG). By elimination of water arises from phosphoenolpyruvate ( PEP). In this last step of glycolysis, the last phosphate group to ADP is transferred, so that pyruvate and ATP occur. On the way from GAP to pyruvate thus two molecules of ATP by phosphorylation of ADP formed per molecule CAP.
Net balance of glycolysis:
Oxidative decarboxylation
→ Main article: Oxidative decarboxylation
The oxidative decarboxylation is a short step, which is, however, essential for the subsequent step. It runs in eukaryotes in the mitochondrial matrix. CO2 removed from the a pyruvate ( decarboxylation) and two hydrogen atoms is controlled by a multi-step reaction mechanism, to NAD transfer ( redox reaction ), and the resulting acetate of the coenzyme A (CoA ) is bound, so that acetyl-CoA is formed.
Balance of the oxidative decarboxylation:
Citric acid cycle
→ Main article: Citric Acid Cycle
The citric acid cycle, also referred to as a citric acid cycle or the tricarboxylic acid cycle, takes place at the matrix of the mitochondria in eukaryotes and prokaryotes in the cytoplasm. It is named after the first intermediate, the citrate, the anion of citric acid.
In the last step of the citrate oxaloacetate formed. This condenses with acetyl -CoA to citrate - by absorption of water and cleavage of coenzyme A. It is therefore the coenzyme A regenerated. Then CO2 is cleaved and hydrogen assumed by the hydrogen carrier NAD (formation of NADH ), so that α -ketoglutarate is formed. The next step is re- cleaved with the aid of coenzyme A and CO2 in hydrogen transfer NAD. The next steps are only the regeneration of oxaloacetate, so that the cycle can start all over again. This is done through the molecules succinyl -CoA, succinate, fumarate, L- malate.
Balance of the citric acid cycle (runs per molecule of glucose from twice because of 1 mol of glucose 2 mol pyruvate and thus formed two moles of acetyl -coenzyme A are ):
Terminal oxidation in the respiratory chain
Through the process so far 4 ATP are formed. However, the greatest part of the ATP - yield delivers the respiratory chain by oxidation of the bound to the hydrogen carrier NAD and FAD hydrogen atoms with oxygen ( O2). A total of 10 NADH are (2 from glycolysis, 2 from the oxidative decarboxylation and 6 ( 2 x 3 ) from the citric acid cycle ) and 2 FADH2 ( flavin adenine dinucleotide ) are available, so 24 reducing equivalents.
NADH is a two electrons (e - ), thereby the bound NAD hydrogen is released as the proton (H ) and the remainder of the molecule is positively charged NAD NAD . Because the votes so 2 electrons are at a fairly high energy level ( very low redox potential of the redox couple NADH / NAD ), can be transported into the intermembrane space with their help 10 protons from the matrix. This is done as follows: the two electrons of the NADH reduce the first complex ( complex I ) of several enzyme complexes of the respiratory chain that are located between the matrix and intermembrane space of the mitochondrion. Each electron is then passed through redox reactions of an enzyme complex to the next. Due to the transfer of electrons from complex to complex, this process is also referred to as the electron transport chain. By the complex I of the complex III to complex IV and H ions (protons ) are transported out of the matrix in the inter- membrane space. In the intermembrane space arises in this way a high hydrogen ion concentration, whereby a pH below 7 is formed, and forms an osmotic potential. Together are called Chemiosmose The redox reactions and the formation of the osmotic potential: The redox reactions are chemical reactions, the difference in H concentrations of matrix and intermembrane space provides an osmotic potential dar.
The protons will eventually flow back through the membrane-bound ATP synthase from the intermembrane space into the matrix space. This enzyme catalyzes the synthesis of a phosphate residue of ATP and ADP. The inherent in this proton -motive force flow energy is used to the fact that arisen ATP is released from the ATP synthase. The transport of a molecule of ADP from the cytoplasm to the matrix or vice versa, the transport of a molecule of ATP to the cytoplasm is catalyzed by an ATP / ADP translocase. However, the proton gradient is tapped for this transport, so that consumes a proton for the availability of ATP or ADP. This must be calculated for the production of a molecule of ATP at least 4 protons.
By the oxidation of a NADH thus produce 2.5 ATP. Exceptions are the two NADH from glycolysis. These are still in the cytoplasm and have to be transported into the mitochondria. If this is done with the help of the glycerol -3- phosphate shuttles, one gains from these only 1.5 per ATP. Since 8 2 NADH are oxidized incurred a total of 8 × 2.5 2 × 1.5 = 23 ATP. However, when cytosolic NADH brought by the malate - aspartate shuttle in the matrix, it can be reduction equivalent per 2.5 moles of ATP produced analogously. This allows a maximum of 10 × 2.5 = 25 ATP are generated.
With the FADH2 the process runs in the same principle, except FADH2 are at a higher redox potential and thus lower energy level electrons. Meanwhile, electrons can thus be introduced only at a lower energy level standing in the respiratory chain. Therefore, can be pumped from the matrix into the intermembrane space with the help of the electrons of FADH2 only 4 protons. With a FADH2 only 1.5 ATP are formed as a result. Since two FADH2 are oxidized, thereby arise 3 ATP.
The protons and the electrons of NADH and FADH 2 ( 24 in total) are oxidized with 6 molecules of O2, which are transported through the membrane into the mitochondrial matrix to 12 H2O. The electron and hydrogen carrier NAD and FAD can be reduced by incorporation of each 2 - D and back to 2 H NADH or FADH2.
Balance of the respiratory chain:
Energy balance
Since prokaryotes do not have cell compartments, they do not have to spend energy for intracellular transport processes and can 36 to 38 moles of ATP gain of one mole of glucose.