Chaperone (protein)
Chaperones (English chaperones ) are proteins that are newly synthesized proteins "help" to fold correctly. The name was chosen " because they retain immature proteins from harmful contact ".
Function
Newly synthesized proteins must first find its specific, native, functional conformation. This is always created in the primary structure, and smaller proteins can fold spontaneously in the right way. The classic example of spontaneous folding of the ribonuclease. Especially in larger, more complex proteins but are often tools for correct folding necessary, since such proteins in the formation of unwanted, dysfunctional aggregations tend.
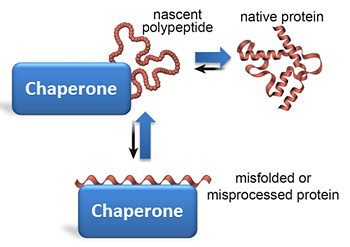
Cells have found a way to minimize the aggregation of newly synthesized proteins from the beginning. For the cell of a complex, highly conserved protein machinery, the chaperones served. These proteins interact specifically with aggregation- prone proteins and thus enter directly into competition with aggregation reactions. The chaperones accelerate this the correct folding and assembly of proteins without being part of the structure itself. Be affected only non-covalent interactions. The following diagram describes roughly the function of the chaperones, where U represents the newly synthesized, still unfolded protein present in a random coil structure. A path now leads to aggregation of the protein (A ), the other way but through the intermediary of the chaperone to the native protein N:
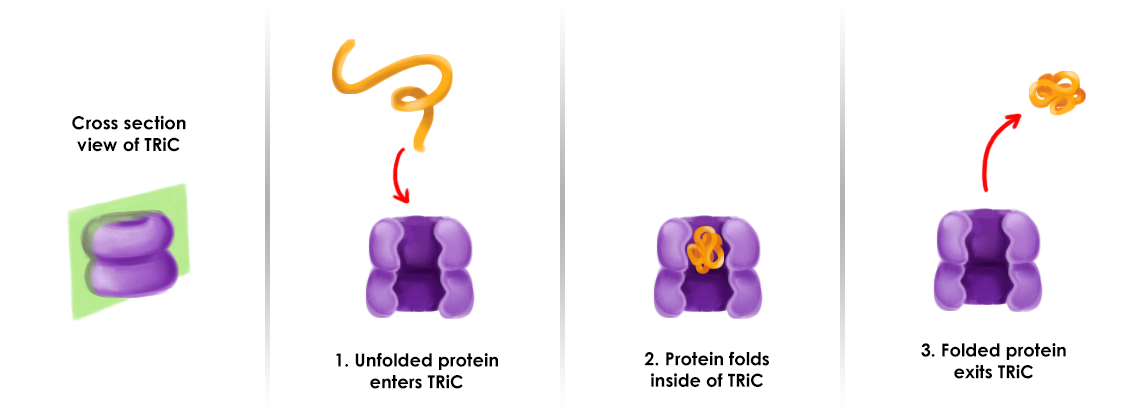
By far the best studied chaperone mechanism, that of the group Hsp60 ( GroEL in bacteria), figuratively donut hole described as hydrophobic: The chaperone is similar to a " barrel " or "donut " with lids on both sides. On the inside of the barrel hydrophobic chains are located, which interact with the hydrophobic regions of the unfolded protein therein and so prevent it from unwanted aggregation. Once the protein has reached its native conformation, the hydrophobic regions are saturated in the protein itself. Under ATP consumption is the "cover" open and released the finished product of the " barrel " or "donut ".
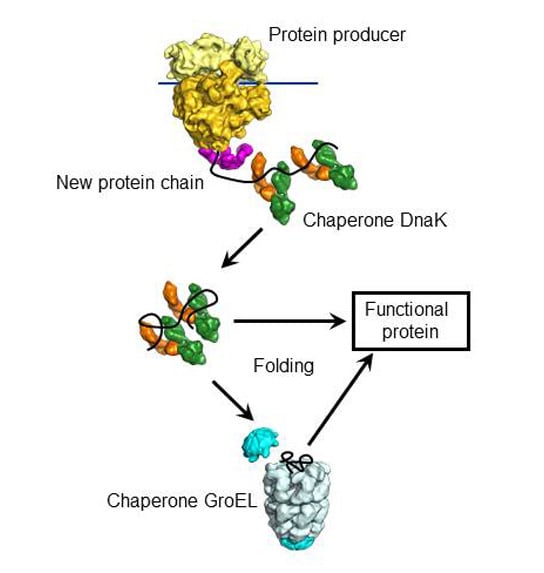
Chaperones are not the only reason why so important because they give newly synthesized proteins, its functional structure, but still have further significance: Since proteins happen only as long amino acid chain without any hydrogen and disulfide bonds, the channel proteins of the cell membranes (eg, in a mitochondrion ), they must be folded back after passing through the cell membrane again, so that they regain their function. This is also an object of the chaperones.
Classification of chaperones
Many chaperones are needed at all to help new chains of amino acids to their physiological secondary structure. The Bakterienchaperon GroEL example helps an estimated half of all medium-sized (30-60 kDa), newly synthesized bacterial proteins during folding. The enormous consumption of ATP, ie on energy, this carries with it underlines the importance of this process.
Other chaperones exhibit at non-physiologically high temperatures to an increased synthesis rate and thus belong to the classical heat shock proteins. However, other factors such as oxidative stress or cell-damaging substances can lead to the accumulation of protein aggregates and thus trigger the occurrence of heat shock proteins. As early as 1988 it could be demonstrated that a clear correlation between the expression of heat shock proteins and the occurrence of thermal tolerance and the ability to tolerate stressful situations, to a certain extent, there is.
In the classification of heat shock proteins sequence homologies and its molecular mass play a crucial role. Using these criteria the current five universal classes could be distinguished from chaperones:
- The Hsp100/Clp-Familie,
- The Hsp90 family,
- The Hsp70 family,
- The small heat shock proteins ( sHsps ).
It can not be traced back to a common Urprotein the chaperones. They represent a heterogeneous class, whose members arose at different times of evolution.