Cyanogen bromide
- Cyanogen bromide
- Cyanobromide
- Bromocyanide
Colorless, volatile needles
Fixed
2.02 g · cm -3
52 ° C.
61.4 ° C
116 hPa ( 20 ° C)
Soluble under hydrolysis in water
Risk
- 92 ppm x 10 min ( LC 50, man, inh. )
140.5 kJ / mol
Template: Infobox chemical / molecular formula search available
Cyanogen bromide is a derivative of hydrocyanic acid (HCN), in which the hydrogen atom is replaced by bromine. Colloquially, it is also called bromocyanide or Campilit.
Occurrence
A biosynthesis of cyanogen bromide was detected in the Nitzschia cf pellucida, a diatom.
Representation and extraction
The preparation of cyanogen bromide is achieved by the reaction of bromine with sodium cyanide in aqueous solution at temperatures below 20 ° C.
A further synthesis starts from potassium thiocyanate or ammonium thiocyanate.
Properties
Cyanogen bromide is still many times more poisonous than potassium cyanide ( KCN). Its extraordinary toxicity is based on the two components and bromine cyanide, which act both by enzyme inhibitory properties as a metabolic poison, as well as toxic to neurons.
Cyanogen bromide is in the form of volatile colorless needles. With a molar heat of formation of 185.6 kJ / mol for Bromcyandampf or 140.4 kJ / mol for the solid is an unstable endothermic compound, the vapor pressure function is correspondingly after Antoine log10 (P) = A- (B / (T C )) ( P in bar, T in K) with A = 3.53667, B = 727.385 and C = -130 052 in the temperature range from 273 K to 313 K.
Add sodium hydroxide solution, the hydrolysis is carried out to sodium cyanate and sodium bromide. The reaction is highly exothermic with a heat of reaction of -235 kJ · mol -1.
At higher temperatures and in the presence of aluminum bromide for the trimerization is carried out cyanuric bromide.
Structure
The BrCN molecule has a linear structure. The bond lengths of the Br - C bonds and C- N bond typical of a single bond, C -Br or C-N triple bond. The bond lengths are 1.160 angstrom for the C- Br bond 1,789 angstroms for the C -N bond. The Bromcyanmolekül is polar. The dipole moment is 2.94 D.
Use
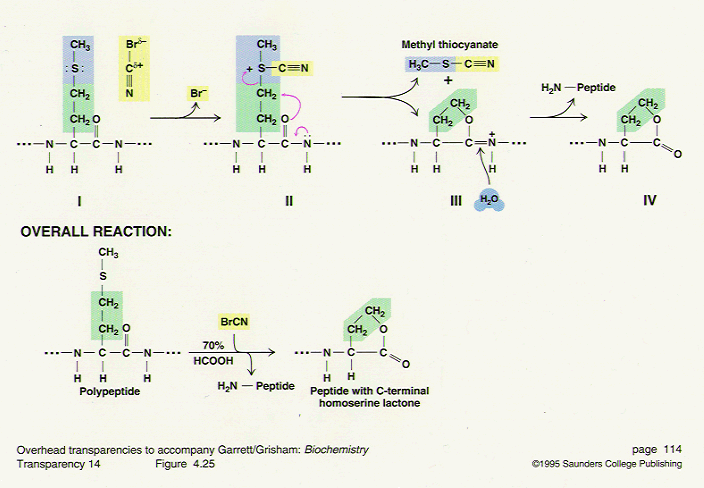
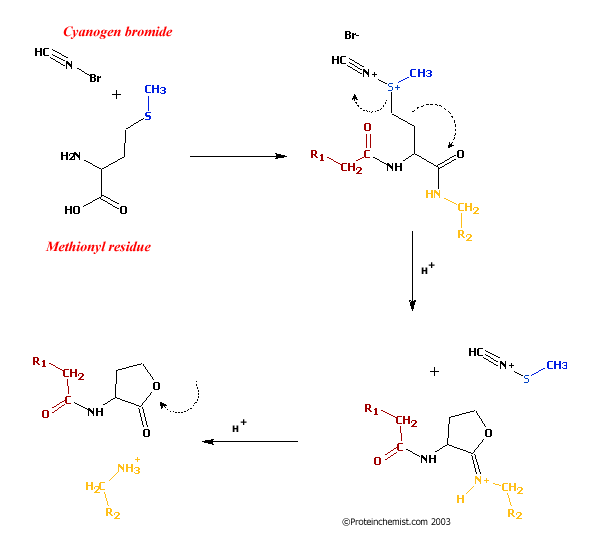
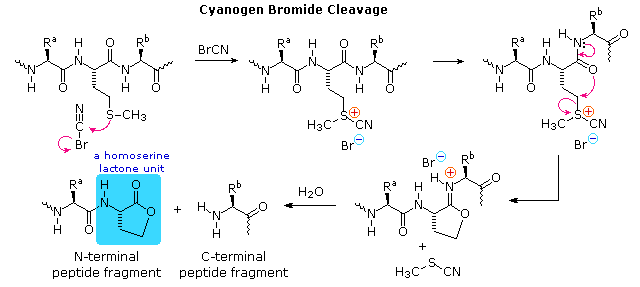
Cyanogen bromide is used primarily in the Goldlaugerei. In biochemistry, it is used for the sequencing of proteins. Cyanogen bromide selectively cleaves C - terminal to the amino acid methionine. The methionine reacts to Iminolacton, then the acid catalysis to homoserine lactone hydrolyzed. Wherein the protein is broken down into smaller fragments, which, for example, the Edman degradation can be analyzed by other analysis techniques.
Safety
Gaseous Cyanogen bromide is hardly absorbed well through the skin on respiratory and digestive tract. Can be hydrolyzed or degraded by contact with water or gastric acid when heated to hydrogen cyanide, hydrogen bromide, cyanuric bromide and cyanogen. On the eyes, skin and mucous membranes cyanogen bromide has a very strong irritant to corrosive. In contrast to cyanide, it is perceived as an irritant when present in the air from 1 ppm; at about 9 ppm is the " tolerance limit ".