Dissociation (chemistry)
Dissociation ( dissociare from the Latin " disconnect" ) is understood in the chemistry of the excited or automatically running process of division of a chemical compound into two or more molecules, atoms or ions. As a measure of the degree of dissociation or dissociation, the dissociation constant is used. The degree of dissociation is the ratio of the dissociated particles for formal initial concentration of undissociated chemical compound. The dissociation energy is the energy that is necessary to cleave a chemical bond.
- 2.1 dissociation in organic chemistry,
Dissociation of gases, thermal, photochemical dissociation
The first methods for molecular weight determination based on vapor density measurements. However, there were sometimes discrepancies, this led to mental conclusions that molecules must be dissociated in the gas phase.
Coined the term dissociation in 1857 by Henri Étienne Sainte -Claire Deville. In the determination of vapor densities of inorganic and organic compounds Cannizzaro, Kopp and Kekulé discovered variations in the molecular weight in the gas phase. Frequently, the gas density was lower than expected. St. Claire Deville could determine a lower density at the phosphorus pentachloride in the gas phase, at the same time he observed a greenish coloration and concluded that phosphorus pentachloride decompose into chlorine and phosphorus trichloride be had. Pebal and Skraup could by heating ammonium chloride in a thin tube through which different gas velocities ( glass tube constriction to measure the effusion, see Thomas Graham ( chemistry ) ) detected with litmus, that the ammonium chloride is dissociated in the gas phase in the ammonia and hydrochloric acid.
Thermal dissociation run much slower than electrolytic dissociation in the rule. An example of a thermal decomposition provides dinitrogen tetroxide that is present at -10 ° C in the form of colorless crystals. Upon heating the molecule dissociates into the intense brown-red colored nitrogen dioxide:
This reaction is reversible. On cooling, the sample is discolored because of recombination to dinitrogen tetroxide again. Dissociations are particularly for macromolecules already at relatively low temperatures.
Upon heating of peroxides or azo compounds, which dissociate thermally bonds already at about 150 ° C, radicals. Radical can be determined by electron spin resonance spectroscopy (ESR ) spectroscopy.
Photochemical dissociation
Through the development of spectroscopic methods and new findings on the bonds in molecules were accessible. An important method was the IR spectroscopy. In the IR spectroscopy, vibrations of the atoms in the molecule can be measured by exposure to infrared light of a specific wavelength. Molecules such as hydrochloric take at slightly different wavelengths of energy, the binding gets into vibrations, similar to two bodies - be moved - connected by a wire spring. At very high vibrational energy, the distance between the atoms is growing to be the bond between the atoms is separated.
In addition to the IR - spectroscopy and photoelectron spectroscopy for the detection of ionization of a molecule is used.
By irradiation with UV light of a discrete wavelength, or dialkyl ketones absorb energy - This leads sometimes to a dissociation of the bond in two radicals. These radicals can be determined with the (ESR ) spectroscopy. More recently, the controlled dissociation of single bonds in the molecule by electron Laserimpules and was possible.
Electrolytic dissociation
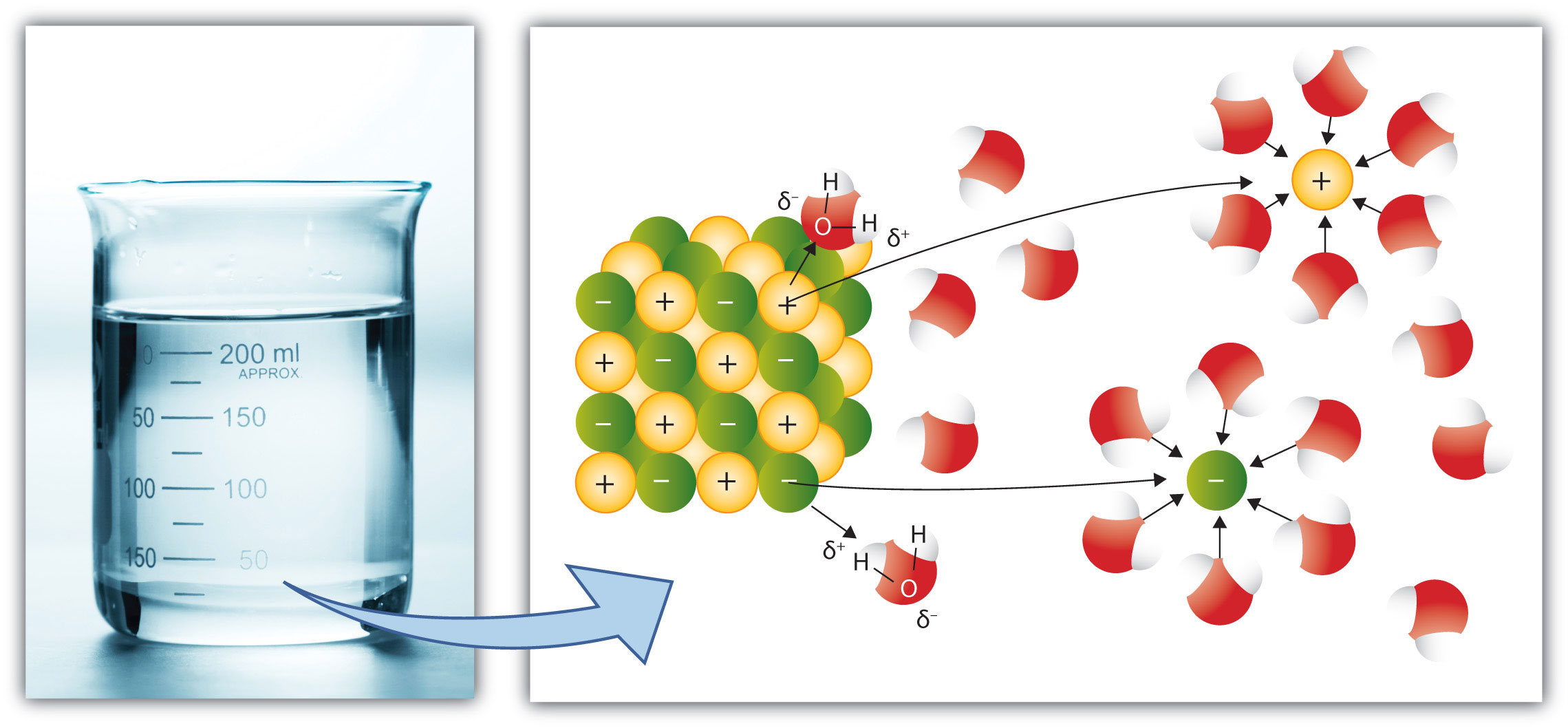
The electrolytic dissociation of the reversible decomposition of a compound in anions and cations in a solvent. The proportion of dissociated ions on the proportion of undissociated and dissociated ions of the same species is called dissociation (see also activity). It depends on the concentration in the solvent. At very high dilution, the acetic acid is completely dissociated in acetate and hydronium ions at high concentration, there is still a large proportion of undissociated acetic acid. The dissociation or more precisely the degree of dissociation of salts or organic molecules can by electrical conductivity measurements ( conductometric ) and are determined by pH measurements of aqueous solutions.
Sodium chloride ( NaCl), which is dissolved in tap or distilled water, dissolves in the form of positive sodium cations and negative chlorine anion. These ions are dissociated, ie, spatially separated from each other and with an electric charge before. Such solutions are called electrolytes. NaCl is strong electrolyte, because the ion almost completely separated present in the solution. Vinegar, comprising the ingredients of acetic acid and water, is also partially in the form of ions, as an oxonium ions and acetate anions before. Acetic acid is not completely dissociated into ions, only about 0.3% of the acetic acid molecule ( in 1 mol / L ) are present in dissociated form. Acetic acid is a weak electrolyte. Depending on the type of solute particles one finds all transitions between strong and weak electrolytes.
In the electrolyte, the equilibrium constant of dissociated products to undissociated precursors from the degree of dissociation and the law of mass action (MWG) can be determined. Wilhelm Ostwald formulated, that the product of active mass concentrations of the dissociated particles (with acetic acid, the Acetationkonzentration multiplied by the Hydroniumkonzentration ) by the concentration of undissociated mass particles ( undissociated acetic acid) always yields a constant. This constant is called the dissociation constant ( obsolete: affinity constant) and it is used for example for the determination of pK a values of acids. The equilibrium constant Ka (a Abk for acid ( engl) = acid), or as negative logarithm ( - logKA = pKa), the proportion of dissociated ions for each concentration can be accurately determined.
It is Ka > 1 for strong electrolytes (salt in water).
In the case of acetic acid in water, the equilibrium lies to the left. It is Ka < 1
If the gas is hydrogen chloride ( HCl ) is introduced into water, an electrolytic solution is called hydrochloric acid forms:
If the gas is ammonia (NH3 ) into water forms as a cation NH4 and the anion OH
The equilibrium reactions of these last three examples are also called proteolysis and is described there in more detail. This behavior makes acetic and hydrochloric acid to form acids. The behavior of ammonia makes ammonia a base. The electrical conductivity of these solutions is the experimental proof of the formation of free mobile anions and cations.
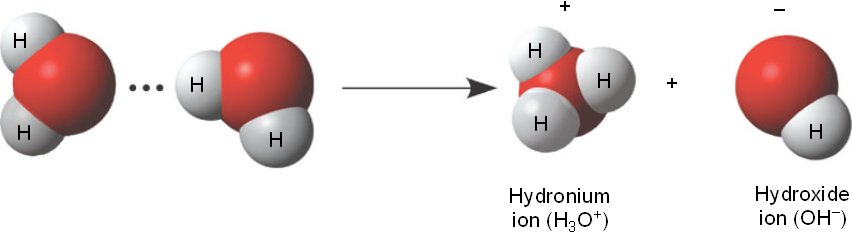
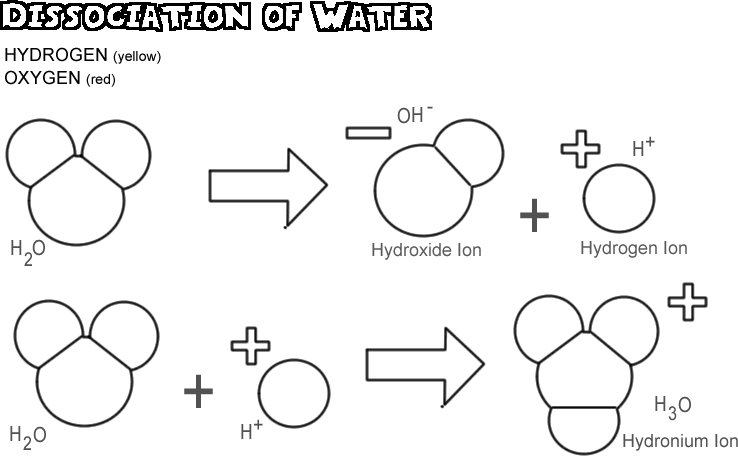
A very important special case is the dissociation equilibrium of pure, distilled water under exclusion of air. Water dissociates to a very small share in the hydroxide and hydronium ions. Kohlrausch and Heydweiller determined the conductivity of distilled water in 1894 to 0.06 · 10-6 Ohm -1. From the conductivity and the knowledge of the boundary conductivities (see equivalent conductivity ) for hydronium and hydroxide ions can be the equilibrium constant, the ionic product of water calculated. It has a value of CW = 10-14 mol2/L2 or pKW = 14 From a knowledge of the concentration of the electrolyte and the corresponding pKa values from tables can easily be determined pH and Dissoziationgrad for each electrolyte.
When loosening of exactly 100.0 g of a 98 % sulfuric acid in exactly 962.7 g of distilled water, the concentration of sulfuric acid is exactly 1 mol / L. Taking of the sulfuric acid solution 106.38 g dissolves in 900 mL of distilled this water, this solution has a concentration of 0.1 mol / L, is orders of 10.64 g of the first solution and dissolution in 990 mL of distilled water the concentration of 0.01 mol / L, analogue can be prepared a 0.001 mol / L aqueous solution of sulfuric acid. The sulfuric acid can be dissociated in water, ie the hydrogen atoms split off as positively charged oxonium ions from the sulfuric acid and as counter-ions to form hydrogen sulfate ions or sulfate ions. The strength of the dissociation is determined by the pKa value. Acids with a negative pKa value are always completely dissociated. The smaller the pKa, the stronger an acid is dissociated.
Suppose that 0.1 molar sulfuric acid would be completely dissociated at this concentration, both hydrogen atoms of the sulfuric acid would have to be split and the Oxoniumkonzentration would have 0.2 mol / L amount. The equivalent concentration (or normal ) of the sulfuric acid would be: 2 * 0.1 mol / L = 0.2 mol / L. Based on 1000 g of water molecules could from these 3.6 g of water molecules are present as oxonium ions. The pH was thus
.
Analog would be obtained for Oxoniumkonzentrationen of 0.02 mol / L: pH = 1.7 and 0.002 mol / L: pH = 2.7. However, the following pH values were measured: 0.2 mol / L pH = 1.23, 0.02 mol / L pH = 1.94, 0.002 mol / L pH = 2.78. In fact agrees with the measured pH halfway to 0.002 mol / L solution. Only there is the sulfuric acid before completely dissociated. In the more concentrated solutions can be approximated formula:
( C is the concentration in mol / l * equivalent number ) are used according to the Ostwald dilution law to determine the pH dependency. For the sulfuric acid thus obtained 0.2 mol / L: pH = 1.30 ( measured: 1.23 ), 0.02 mol / L: pH = 1.80 ( measured: 1.94 ). A high proportion of sulfuric acid is consequently higher concentration than hydrogen sulfate (over 40% in a 0.1 molar sulfuric acid ) rather than two-fold negative sulfate before and this explains the differences in the pH measurements. For hydrochloric acid and nitric acid, the logarithmic concentration dependence is valid for the pH determination according to the first relationship, for caustic soda in an analogous manner for the hydroxide concentration. In titrations with sodium hydroxide or with further dilution of the solution, the Schwefelsäureteilchen are dissociated and the correct total concentration is obtained.
Weak acids and bases can act as a buffer (chemistry). The pH of a solution remains fairly constant and equal to the pKa of the corresponding acid, when a weak acid and its anion are present in approximately the same concentration in the solution. Important biological buffers are solutions of carbonic acid and bicarbonate and dihydrogen phosphate buffer, they shall see in biological systems for a constant pH. Acid-base titration with a suitable pH indicator or a pH electrode to be used in such solutions to determine the concentration of this acid and base correctly.
Salts can dissociate more or less strong. Salt solutions at a high concentration will exhibit different properties than would be expected when the physical measurement value of the diluted solutions would be extrapolated to solutions of high concentration. In the physical properties, it may, for example, the freezing point, boiling point or the electrical conductivity. To describe a solution but now has a linear dependence to a hypothetical concentration ( activity), the concentration of a solution must be multiplied by a factor, the activity coefficients. In order to describe the concentration-dependent conductivity of the equivalent conductivity coefficient is used according to the coal noise law, this differs considerably from the activity coefficients.
In the so-called real or permanent electrolyte, the ions already in the solid state ( ionic lattice ) are present. Indeed, in the solid salt to grid Na and Cl - ions. On dissolving the salt in water are now formed in the water freely moving ions. In the dissociation of salts in the ion rather high lattice energy of the crystal is applied by hydration during solution process.
In the so-called potential electrolytes lie with the pure substances have no ionic bonds. As a pure substance they are insulators. During the introduction of the pure substances (AB) in a solvent, the formation of ions by a chemical reaction between the solute and solvent;
Prerequisite for such a reaction is a polar bond between the parts A and B of compound ( AB) and a polar solvent. For example, pure acetic acid is added to water form as cations H3O , the anions H3C -COO-
Not only in water ions are dissociated. An almost equal high degree of dissociation is also observed in polar organic solvents such as formamide, acetonitrile or nitromethane. The decisive factor for the dissociation in organic solvents is the dielectric constant, such as Walter Nernst found out.
Dissociation in organic chemistry,
In the organic chemistry, knowledge of the dissociation of great importance.
Many organic reactions are possible only if carboxylic acids, hydroxy groups are present as anions, thus conversion processes such as alkylation can be performed. Hammet investigated the dissociation of the organic bases and the carboxylic acid in water and various solvents. The first study on the formation of organometallic compounds were carried out by Conant and Wheland. In 1965, then put on a Cram acidity scale ( MSAD scale) for different hydrocarbon molecules, EM Arnett had 1963 intended for esters, amides, thiols, amines, phenols dissociation constants. Because of this Aciditätsskalen organic chemists can easily assess which base is necessary for a substance implementation.
History electrolytic dissociation
Already in 1795, a charge separation of particles was Volta after the discovery of electrochemical electricity by Galvani contemplated by different metals. Grotthus developed in 1805 a theory for charge separation of water molecules. Berzelius and Daniell were concerned to salts in water, they assumed that the salts are there separated into positive and negative charge carriers. Faraday coined the term of the ion, but he had not the current notion of a localized charge on an atom or molecular moiety in solution. Rudolf Clausius believed that the charge carriers are not fixed, but perform oscillations. He realized even then the possibility of dissociation of electrolytes. Since the first stream was only obtained from electrochemical batteries and the basic laws of electricity and electronics have been developed with these batteries, had the question to the electrolyte and the conductivity of the solutions a high priority.
Basic work on the actual dissociation were, however, only by Friedrich Wilhelm Georg Kohlrausch ( producing a conductivity meter with AC for liquids, formulation of a law for strong electrolytes, carbon intoxication square-root law ), Jacobus Henricus van ' t Hoff ( Osmotic pressure as a function of the number of particles ), Svante Arrhenius (works on the conductivity with Kohlrausch's conductivity meter and postulate the dissociation of salts in positive and negative charge carriers, founder of dissociation ) and Wilhelm Ostwald (dissemination of Arrhenius ideas, application of the law of mass action to the dissociation and formation of the important law for weak electrolytes, Ostwald cal dilution law ) made .
A mathematical theory of dissociation was later developed by Peter Debye and Erich Hückel. With this Debye- Hückel theory can be calculated mathematically from known boundary conductivities, the degree of dissociation of the electrolyte. However, the model is only for low electrolyte concentrations (up to 0.01 mol / l ) are suitable.
Max von Laue showed by X-ray structural analysis that ions are also present in solid salts.