Electrophilic aromatic substitution
An electrophilic aromatic substitution - abbreviated as SEAr - is an electrophilic substitution reaction of an aromatic compound. While aliphatic substitutions are often nucleophilic nature, aromatics are conditionally preferentially attacked by their electron-rich π - system of electrophiles. As a rule a bound to the reacting aromatic hydrogen atom is replaced by the electrophile. The electrophilic aromatic substitution is a multi-stage reaction.
- 4.1 directing effects
- 4.2 second substitution
- 4.3 third substitution
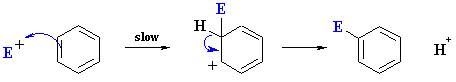
Reaction mechanism
The reaction mechanism in Figure 1 describes the general flow of an electrophilic aromatic substitution. The aromatic 1 interacts with the electrophile E . One speaks of the formation of the π - complex 2a. From this, or directly from the starting materials formed under suspension of aromaticity of one of the resonance-stabilized σ - complex 2b, which is also called arenium ion or Wheland complex. Deprotonation of this complex under the re-aromatization of the system takes place, and the final product 3 is released. The formation of a π - complex is not essential for the explanation of the reaction is necessary.
Electrophile
As the electrophile E a wide range of compounds can be considered that often the products of a reactive electrophilic aromatic substitution reactions are upstream. In the context of electrophilic substitution, the following electrophiles of importance:
- The proton
- Polarized halogens, for example, Bromination or iodination: Br2 → Brδ Brδ -
- Hypobromous acid HBrO
- Iodine chloride ICl
Some of the electrophiles are positively charged and can directly replace the leaving group. In others, the positive charge must be generated by bond cleavage. The coordination of a Lewis acid to the negative end of the electrophile increases the polarization of the bond and accelerates the electrophilic substitution. This happens, for example, in the bromination with bromine Br2 in the presence of iron (III ) bromide, FeBr3.
The leaving group
As a rule, the leaving group is a proton. Furthermore, examples of carbocations sulfonyls and silyl groups are known.
Kinetics of the reaction
Figure 2 shows the reaction coordinate of the reaction. For the two-stage there are many documents. On the one hand it is possible occurring as an intermediate arenium ion 2b to isolate in pure form and characterized. To succeed in the representation of the σ - complex 4 from mesitylene and ethyl fluoride in the presence of boron trifluoride in ether at -80 ° C. On the other hand, one can calculate about the investigation of isotope effects kinetics of the reaction, which suggests a two-step mechanism. After the formation of σ - complex of the rate-determining step.
Substituent
Already present on the aromatic substituents exert a large influence on both the reactivity of the aromatic ring and thus on the rate of electrophilic substitution, as well as on the position of a second substitution from ( directing effects). Is the electron density of the aromatic ring increases, so also increases its reactivity towards electrophiles. This increase may be caused by mesomeric and / or inductive effects - this is known as M- or I- effect. An adjunct sign indicates whether the substituent is the electron density of the ring increases ( M / I effect ) or decreased ( -M/-I-Effekt ).
The inductive effect is due to the fact that electron-withdrawing substituents destabilize the protonated ring. The mesomeric effect is due to the fact that the substituent has lone pairs, through which he can increase the electron density by resonance in the ring or decrease.
Directing effects
In addition to influencing the reactivity of the aromatic compounds of the first deputy acts directing the entry position of the second substituents: Electrophilic aromatic substitution reactions with I / M substituents provide regarding its position mainly ortho -and para -substituted, -I/-M-Substituenten contrast, meta- substituted products.
For an explanation of this effect we consider the resonance structures of either the aromatic reactant or the σ - complex. In fact critical for the preferred second substitution, the height of the first transitional state between the reactant and the σ - complex. When considering the resonance structures of the starting material to argue with a relieved attack of the electrophile to electron-rich atoms of the ring and a consequent lower transition state. When considering the σ - complex, it is believed that a more stable, ie lower-energy σ - complex was achieved via a lower transition state. Generally speaking, one can say that electron- donating groups - ie electron donor groups - such as - CH3 or -OCH3 ortho / para- directing effect, while electron-withdrawing groups - so-called electron - as - NO2 or - CO2H function meta- directing.
Second substitution
The following examples show different ways the second substitution:
Nitrotoluene
In the nitration of toluene with nitric acid to the I effect of the methyl group is crucial so that arise for the management of the second substituents, as the main products of o- nitrotoluene with 65 % and p- nitrotoluene with 30 % m- nitrotoluene contrast, only 5%.
Dinitrobenzene
Here, the - I effect and the - M effect of the nitro group of nitrobenzene to cause 93% of a Directorate in the meta position. Ortho -and para- positions only occur to 6, or 1%.
Third substitution
With an electrophilic aromatic substitution with two or more substituents already present at the site of the reactivity and substitution of most of the combined effects of the substituents can be derived. Often this is very simple, when the effects of the substituents reinforce each other or all free positions are equivalent. If the directing effects of different substituents would lead to a substitution at various points, usually determined by the most activating substituent acting, where the substitution takes place. If two positions by I- effects are similar preference of alkyl substituents, steric effects gain in importance and the substitution takes place at the more accessible location.
The figure shows the nitration of nitrotoluene to dinitrotoluene. First, a π - complex between the nitronium ion NO2 and the aromatics forms, then they respond to a signified as σ - complex carbenium ion, the resonance structures are shown in square brackets. This intermediate product is in the lower reaction path in the less stable than unsubstituted aromatics, as the positive charge near the electron-withdrawing nitro group. The above intermediate product is stabilized, as the positive charge near the electron-donating methyl group. The top product is formed preferentially.
Important electrophilic substitution reactions of aromatics
- Aminomethylation
- Azo coupling
- Blanc reaction ( chloromethylation )
- Friedel-Crafts acylation of
- Friedel -Crafts alkylation
- Halogenation
- Houben -Hoesch synthesis
- Hydroxymethylation
- Gate man - synthesis
- Gate man - Koch synthesis
- Kolbe -Schmitt reaction
- Nitration
- Sulfonation
- Vilsmeier -Haack reaction
- Diazotization