Lead(II) hydroxide
- Lead hydroxide
- Causticized Cerussite
White amorphous powder
Fixed
7.41 g · cm -3
145 ° C ( decomposition)
- Slightly soluble in water
- Insoluble in acetone
- Soluble in dilute acids and bases,
Risk
Template: Infobox chemical / molecular formula search is not possible
Lead (II ) hydroxide is a chemical compound of the lead from the group consisting of the hydroxides.
Production and representation
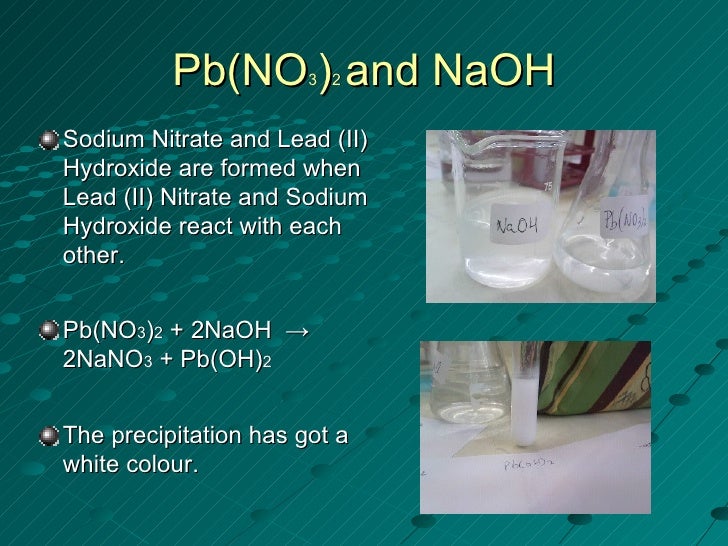
Lead (II ) hydroxide may be obtained by reaction of sodium hydroxide or potassium hydroxide with a lead (II ) nitrate solution.
However, according to other sources while there is no pure lead (II ) hydroxide, but a mixture of Bleioxidhydraten and lead nitrate oxide hydroxide. It is precipitated at the reaction of lead (II ) salts, so instead of the expected lead (II ) hydroxide, the white lead synonymously used for (II ) oxide hydrate PbO · x H 2 O ( x <1). With careful hydrolysis of lead ( II ) acetate solutions obtained a crystalline product with the formula PbO 6 · 2H2O = Pb6O4 (OH ) 4
Lead (II ) hydroxide is also formed during contact of lead with oxygen and water carbonatarmen.
Properties
Lead (II ) hydroxide is a white amorphous powder amphoteric, which dissolves only slightly in water. It dehydrated from a temperature of 130 ° C and decomposes above 145 ° C for lead (II ) oxide and water.
With carbon dioxide, it is lead (II ) carbonate
And lead salts thereof with acids ( sulfuric acid, for example with lead (II) -sulfate )
Upon dissolution in strong alkalies it dissolves with the formation of a hydroxo complex ion [Pb (OH ) 3] - and the formation of plumbates.
Use
Lead hydroxide is used for the preparation of porous glass and in the electrolyte in a sealed nickel-cadmium batteries. It continues to be used in the recovery of uranium from seawater, and as a catalyst for the oxidation of cyclododecanol.




_sulfate.jpg)