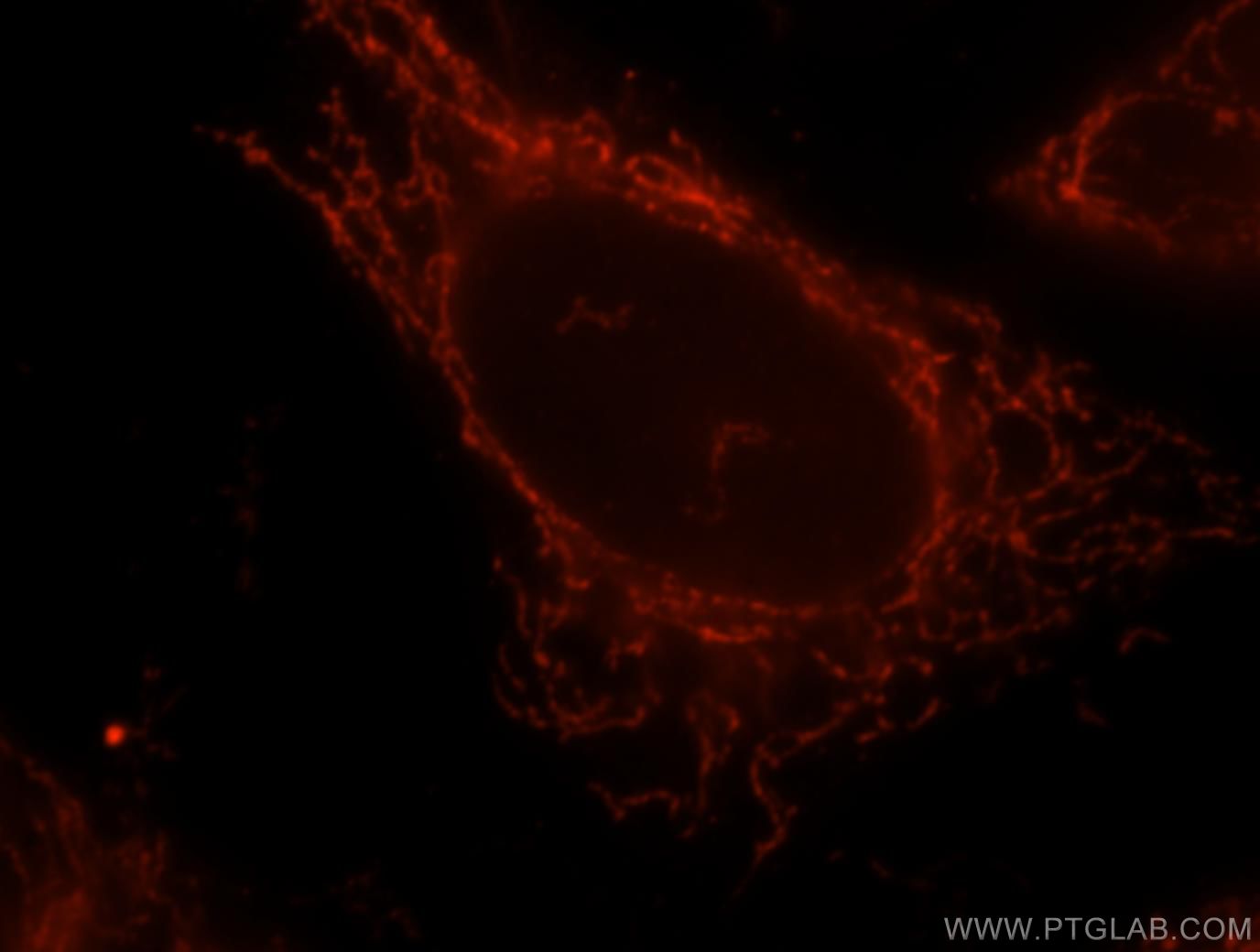
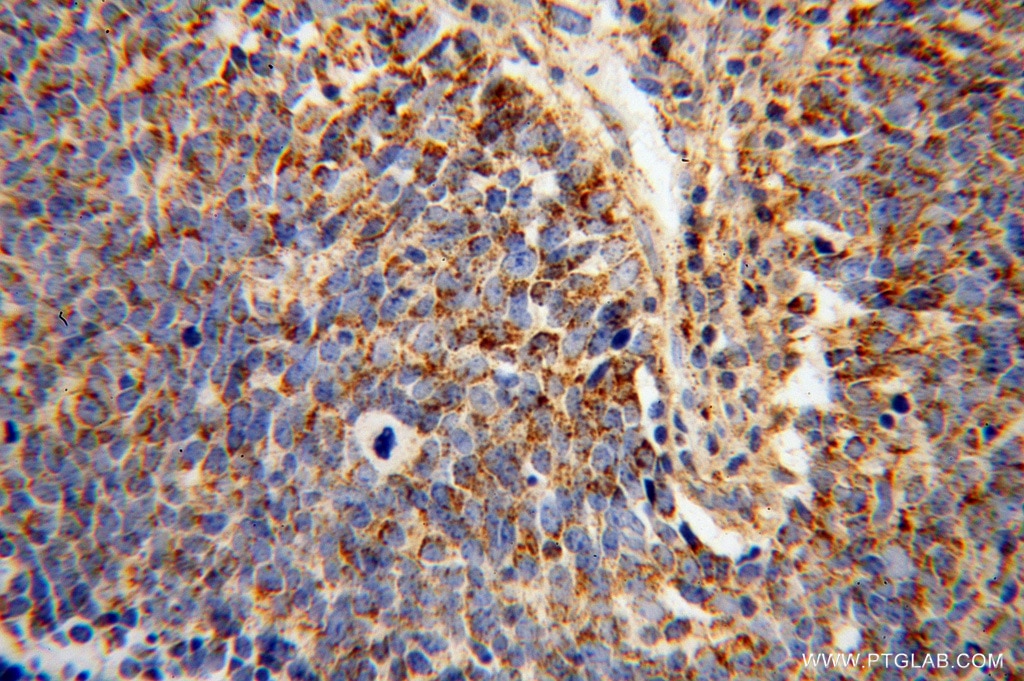
OGDH
- OMIM: 613022
- UniProt: Q02218
α -ketoglutarate dehydrogenase E1 is the enzyme in bacteria and eukaryotes, catalyzes the E1 subunit of α -ketoglutarate dehydrogenase complex, the decarboxylation of α -ketoglutarate. This reaction is part of the citric acid cycle. In addition, α - Ketoadipat decarboxylated, what is needed in the degradation of the amino acid lysine. The protein complex is localized in the mitochondria. Rare mutations in OGDH gene can lead to E1 deficiency and for this rare Ketoglutarazidurie.
Catalyzed reaction
Decarboxylation of ketoglutarate finds the thiamine rather than catalytic center, which forms a bond with the atom ketoglutarate, succinyl so that thiamine pyrophosphate is formed with elimination of CO2.
CO2 (R = OOC- CH2 -CH2-)
This succinyl ( syn. succinic acid ) of the TPP is taken from the α -lipoic acid ( oxidation). It is covalently bound to the lipoate DSLT subunit. The result is S- succinyl- Hydrolip ( oat / onamid ).
(R = OOC- CH2 -CH2-)
Thus, the reaction is equivalent to the decarboxylation of pyruvate by pyruvate dehydrogenase E1 runs.