Period 6 element
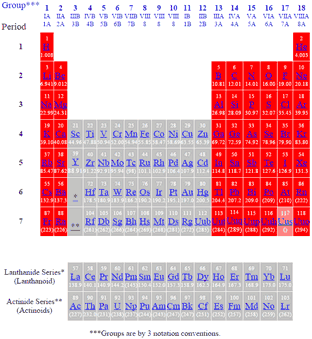
The sixth period of the periodic table of the elements contains all the chemical elements that have exactly six electron shells in the atom. The innermost (first) electron shell is fully occupied and has two electrons. The second electron shell with eight electrons, and the third electron shell with 18 electrons, are also fully occupied. The fourth electron shell has at least 18 electrons and can accommodate a maximum of 32 electrons. The fifth electron shell has at least 8 electrons and can hold up to 18 electrons. The outermost (sixth ) electron shell, also called valence shell, can accommodate between one and eight electrons. Thus, a total of 32 chemical elements are in the sixth period. The lanthanides are a special group because within this period.
Excerpt from the Periodic Table
Magic Number
The chemical element with atomic number 82 lead has in the ground state of the nucleus is more stable than neighboring nuclides. This particular order number is called the magic number.
Number of electrons in the electron shells
List
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8
- Group of the periodic table
- Period 6 element






.svg/2000px-Periodic_table_(polyatomic).svg.png)

