Piperidine
- Hexahydropyridine
- Pentamethylenimin
- Azinan
Colorless liquid with an ammoniacal odor
Liquid
0.86 g · cm -3
-10 ° C
106 ° C
33 hPa ( 20 ° C)
- Miscible with water
- Soluble in ethanol, diethyl ether, benzene, chloroform
1.4530 (20 ° C)
Risk
Template: Infobox chemical / molecular formula search available
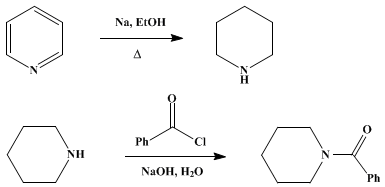
Piperidine ( systematic name after the Hantzsch - Widman System: Azinan ) is a colorless, ammonia-like smelling liquid. According to its chemical structure, there is an annular secondary amine.
History
Piperidine was first isolated in 1819 by the Danish chemist Hans Christian Ørsted from pepper. 1894 succeeded Albert Ladenburg and Scholz, the first total synthesis of piperidine.
Occurrence
Piperidine is a structural component of the alkaloid piperine, which is found in black pepper ( Piper nigrum).
Synthesis
Piperidine obtained quantitatively by catalytic hydrogenation of pyridine by electrochemical reduction of pyridine or heating Pentamethylendiamindihydrochlorid, to obtain the hydrochloride.
( RaNi Raney nickel as a catalyst for the hydrogenation reaction )
Responsiveness
Piperidine is a base ( pK a = 11.12 ). With heavy metal salts, it forms complexes.
Use
Piperidine. Solvent, an intermediate in the synthesis of pharmaceuticals, pesticides, and fragrances, as a curing agent used for epoxy resins, as a catalyst for condensation reactions, as the rubber auxiliaries, and as a reagent for aldehydes, gold, cerium, magnesium or zirconium
Since piperidine can be used for the production of non -marketable anesthetic phencyclidine, it is listed in the Appendices of the Commodities Control Act. Trade, Ein-/Ausfuhr and production of less than 0.5 kg, not notifiable.
Hindered piperidine derivatives (usually 2,2,6,6 -tetramethylpiperidine derivatives) serve as technically and economically significant light stabilizers for plastics and are also referred to as HALS ( Hindered Amine Light Stabilizers of English ).