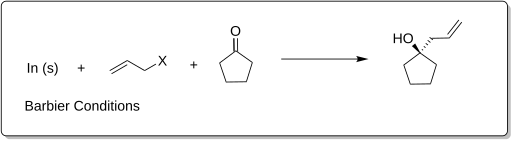
Barbier reaction
The Barbier reaction, also called barber rearrangement reaction is a name in the field of organic chemistry. It is named after the French chemist Antoine Philippe François Barbier (1848-1922), the supervisor of Victor Grignard. It was in 1898, so only two years before the Grignard reaction, released for the first time.
Survey
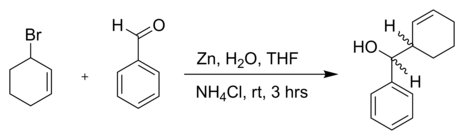
The Barbier reaction has far-reaching applications in organic chemistry, such as in the synthesis of secondary or tertiary alcohols. In this case a halogenated with chlorine, bromine or iodine organic radical reacts ( alkyl, Arly, benzyl or allyl radical ) to a metallic surface consisting of magnesium, lithium, aluminum, samarium, antimony, bismuth, cadmium, gallium, indium, manganese, tin or zinc, etc. directly in the presence of an aldehyde (R2, R3 = H, Organylgruppe ) or ketone (R2, R3 = Organylgruppe ) with the carbonyl group. The hydrolysis then leads to alcohol:
One of an allyl halide newly formed Grignard reagent reacts in a secondary reaction with further allyl halide to form dimers. The Barbier reaction is a way to circumnavigate this problem:
Mechanism
The mechanism is shown here as an example with allyl bromide, propanal and metallic magnesium.
First, bringing the 3- Bromprop -1 -ene ( 1) on the surface of the metallic magnesium. Characterized the Gringard reagent formed prop-1- enmagnesiumbromid (2). This is ( not shown here ), these forms represent only a small proportion of the equilibrium with an ionic and a radical form in equilibrium. Now Puts you this Gringard reagent ( 2) with propanal, the result is the hex-5 - en-3- olate (3). This reacts with acidic workup for hex-5 -en-3 -ol ( 4).
This reaction is the Grignard reaction is very similar with the difference that it is a one-pot reaction. This means that all reagents are mixed together at the same time, one also speaks of a " one-step reaction." In contrast, the Grignard reagent must first be generated in the absence of Carbonylsubstrates .. The Barbier reaction falls within the category of Nucleophilic addition reactions in the Grignard reaction. When you can work in contrast to the Grignard reaction in aqueous solvents, because the organometallic intermediates are sensitive to protic solvents. For this reason, you can assign the Barbier reaction of Green Chemistry .. also, in contrast to Gringard reaction has the advantage that it provides significantly less toxic halides with the same results. It is preferred for you reaction of allyl or benzyl bromides. It is also suspected that it runs with a single electron transfer (SET, single- electron - transfer).
Examples
The following examples are intended to illustrate the wide application possibilities of the Barbier reaction. The reaction between propargyl and butanal in the presence of metallic zinc provides an alkyne derivative:
Also, the Barbier reaction intramolecularly run, as shown in the following example. In this way correspond substituted molecules can be cyclized using the Barbier reaction. For chromium dichloride and nickel chloride thereby forming previously by a redox reaction, the catalytically active nickel (0).