Beckmann rearrangement
The Beckmann rearrangement reaction is a name in the field of organic chemistry and was named after its discoverer, the German chemist Ernst Otto Beckmann (1853 - 1923), named. The rearrangement is used for the synthesis of carboxamides.
Overview reaction
In this reaction, a reaction catalyzed by acids conversion of ketoximes bzw.Aldoximen into carboxamides done. By further hydrolysis of the Beckmann rearrangement of aldoximes leads ultimately to the amine.
If it is a ketoxime, R1 and R2 are organyl. At one of the remaining groups aldoximes is a hydrogen atom.
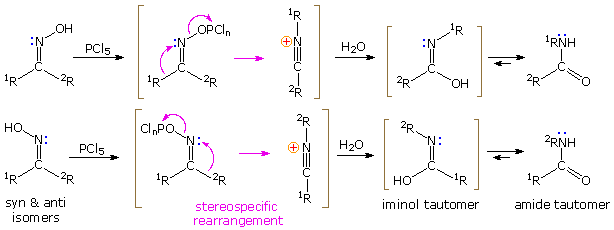
Reaction mechanism
Beckmann rearrangement of the ketoxime starts protonation of the hydroxy group. The water is eliminated and one of the two radicals migrate to the nitrogen. It always moves the group is located trans to the hydroxyl group. After nucleophilic attack of water and deprotonation of the Iminolform which then tautomerizes to amide is formed. Since the oxime group is angled on the nitrogen, are at different residues before R1 and R2 E / Z isomers.
The preparation of nitriles by " drainage " of aldoximes with, for example phosphorus pentachloride, PCl5, is based on an analogous mechanism:
Application
The Beckmann rearrangement has importance in the industrial production of ε -caprolactam, the raw material for Perlon production. Here, cyclohexanone oxime is rearranged in the presence of sulfuric acid: