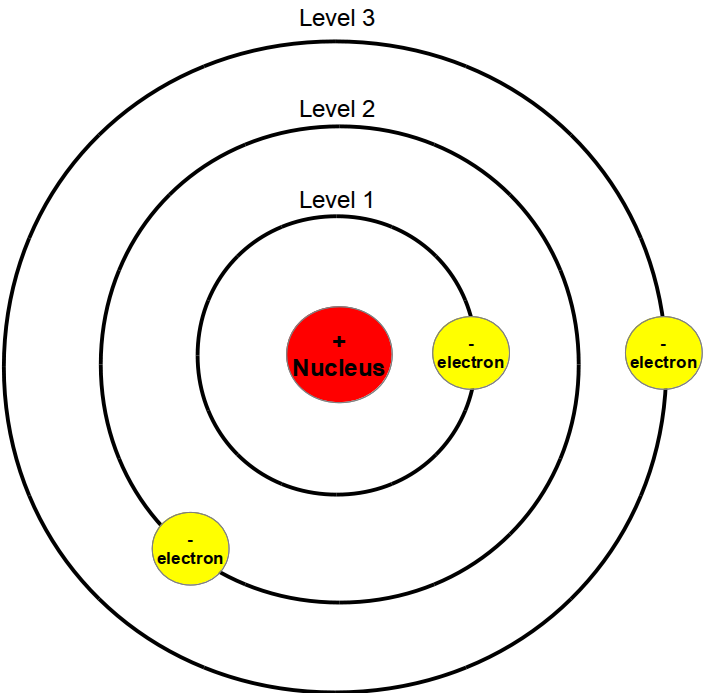
Bohr model
The Bohr model of the atom is the first widely accepted model of the atom that contains elements of quantum mechanics. It was developed in 1913 by Niels Bohr. Atoms are made in this model of a heavy, positively charged nucleus and light, negatively charged electrons orbiting the nucleus on closed paths. Through three postulates continued drilling within the model of classical physics partially suspended. Unlike older models of the atom is the Bohr atomic model, many of the observed properties of the hydrogen atom. On the other hand, many details of spectroscopic measurements of it are not recognized. Chemical bonds can not explain it. The concept of himself is on tight orbits around the nucleus moving electrons in contradiction with the uncertainty principle.
Bohr's atomic model paved the way to understand the construction of the atomic shell. The intuitive idea of electrons orbiting the nucleus like planets, the sun, has shaped the popular image of atoms for decades. They can still be found on logos and cartoons. Quantum mechanical models of the atom from about 1925 see no tracks, but probabilities for electrons.
- 2.1 Bohr's postulates
- 3.1 Size of atoms
- 3.2 Spectral transitions
- 3.3 discrete energy states
- 5.1 Stationary States
- 5.2 atomic size
- 5.3 position of the lines in the spectrum of hydrogen
Overview
Bohr took the Rutherford model of the atom as a starting point. This negatively charged electrons orbiting a positively charged nucleus like the planets orbit the sun in the Copernican system. According to classical electrodynamics an orbiting charge generates electromagnetic waves with which energy is radiated. Consequently, each orbiting electron would lose energy and fall on a spiral path to the nucleus. Stable atoms there could not be thus. However, as there are atoms stable size, the model is refuted in this form.
In order to describe atoms that are stable despite circling electrons, Bohr solved in 1913 partly on the validity of classical mechanics and electrodynamics. He assumed that there are certain paths for electrons in the atom on which they orbit in a stable form the core without generating electromagnetic waves, and that all other paths which are also possible according to classical mechanics, not found in nature. Radiation gives the atom only in the transition of an electron from one of the old tracks in a different from where - has nothing to do with the frequency of the wave generated directly with the frequencies with which the electron beforehand or afterwards in - in contradiction to the electrodynamics again circle moves. But can no more statements are made about the precise sequence of this quantum leap. Bohr broke it with the existing conflicting natura non facit saltus theorem ( " nature does not make jumps "). Overall, he was guided by his strong intuition in the preparation of these principles.
For the selection of stable orbits Bohr has endorsed three postulates. His goal was, the Planck constant as the characteristic of quantum physics to take into account such natural constant in the equations that the results of his model as well as possible reproduce the observed facts of atoms. His model successfully demonstrated that one could derive many properties of atoms by a combination of several exceptions to the classical physics and few, seemingly simple new conditions. These results provide the data of the hydrogen atom (in the context of the then possible accuracy ) good again: its size, the characteristic wavelengths of the line spectrum, its ionization energy. This agreement with experimental results legitimized the sometimes revolutionary postulates. Therefore, the model played a major role in the further development of atomic physics. Because of its clarity, after the development of quantum mechanics in 1925 could not be sustained in the otherwise much better models, the Bohr model is still used today in many cases as a basis for the qualitative description of atomic processes.
Predecessor and successor models
As a precursor to the Bohr model of the atom model by Arthur Erich Haas ( 1910) can be referred. Haas pursued the idea to understand the Planck constant of the properties of the electrons and the atoms ago. He walked out of the then widely accepted Thomson 's atomic model and adopted for the simplest atom, an electron circles within a positively charged sphere the size of the atom. Then he put the energy difference between the rest position of the electron at the center and its maximum circular path - arbitrarily - with the energy of a photon is equal which, according to the equation of the cut-off frequency of the spectral lines of the Balmer series. He received from an equation which is in good agreement with the known radius of the hydrogen atom of the orbit radius. Conversely, this equation allows to calculate the radius of the cutoff frequency of the spectral lines, and thus the ( Rydberg constant ). The same equation led Bohr in his model in 1913 ago, but for the smallest possible orbit of the electron ( during the Balmer series in his model a 4-fold greater web part ). After all, showed the atomic model of Haas that Planck's constant is a physical constant that may be suitable for the calculation of atomic quantities, if you - even at the seemingly arbitrary way - fits into a physical model of the atom.
With respect to the quantization had been proposed in 1912 by John William Nicholson, in principle, the quantization of the angular momentum in steps be preferable to the quantization of energy in steps. In support of him was that the angular momentum of circular orbits is just the ratio of energy and angular frequency as in Haas'sche model on the one hand and on the other hand is a multiple of. This meaning of the Planck constant proved in fact to be essential (see Planck's constant # angular momentum ). However, the absence of further explanation, neither the value nor the ideas of Haas by Nicholson to be useful models of the atom were adopted.
Direct sequel to the Bohr model was from 1916, the bohr - Sommerfeld model of the atom. In it were included according to the suggestion of Arnold Sommerfeld elliptical orbits to gain more and more accurate results after improved experimental methods have increasingly provided small deviations from the predictions of the Bohr model.
The model
Bohr's postulates
Drilling followed up on Rutherford's idea of 1911, after which an atom consists of a positively charged, very small and heavy atomic nucleus, which is surrounded by a number of electrons. He examined periodically circulating movement of a single electron, as follows from the formula of classical mechanics, when the force between the core and electrons resulting from the electrostatic attraction. In order to adapt this model to the observed properties of the hydrogen atom, he extended it to three postulates:
In the first two postulates Bohr formulated to apply only limited to the level of atoms, the laws of classical mechanics and electrodynamics. Unlike in classical mechanics no continuous transition, but a quantum leap is assumed between two states. In the detailed calculation it sets the first postulate so that it assumes circular orbits and takes in direct contradiction to the theory of electrodynamics that the electrons in the circulation do not lose energy in the form of electromagnetic radiation. The second postulate is contrary to the electrodynamics, because the frequency of the generated wave does not have to match the rotational frequency of the wave generating particle. ( With the parameters of orbit radius, energy, rotational frequency ) and the emitted radiation to derive a result ( and with the help of another, but absurd and false additional assumption ), he succeeds in completely new formulas for the relationship between the electron motion ( frequency parameter ) that the now Rydberg formula look like.
To further select from these general formulas to the right, he uses in his third postulate for the first time discovered by him ( but later so designated ) correspondence principle between classical and quantum physics: Despite the stark opposites as they are stated in the first two postulates, there must be in the new quantum physics a smooth transition from the familiar and proven classical physics. This yields ( after some calculation) from the third postulate that the stable electron orbits are characterized in that the orbital angular momentum of the electron is an integral multiple of the reduced Planck's constant:
This is also sometimes referred to as the third Bohr postulate, because it allows a rigorous derivation of the equations of the Bohr model of the atom without the correspondence principle or such additional assumption is wrong effort (see below Mathematical formulation). Bohr himself later referred to only the first two assumptions as its postulates.
Confirmations
Bohr's atomic model was able to explain a range of physical measurement results of the nascent atomic physics. In subsequent experiments carried out with higher accuracy, however, also showed significant deviations between model and reality.
Size of atoms
The computed with the few basic assumptions of the model diameter of atoms is for many elements in the correct order. In particular, they agreed roughly correspond to the same time of Max von Laue and William H. Bragg first experiments performed for X-ray diffraction. The small but finite size was a key property of the atoms in the still vague ideas about the structure of matter. Therefore, the ability of the Bohr model to derive the size of general assumptions considered a success.
Spectral transitions
In the first half of the 19th century spectral lines were discovered in the hydrogen atom. For the position of the lines within the respective series Johann Jakob Balmer and Johannes Rydberg were based on measured line spectra in 1885 and 1888 numerical formulas specify ( Balmer series, Rydberg formulas ). However, the physical background of these formulas remained a mystery for almost thirty years. The introduced by Bohr spectral transitions of electrons from one shell to the other allowed, the Balmer and Rydberg formula derived from general principles. They were also an intuitively plausible picture of the processes in the atom. A series corresponds to the transitions of electrons higher levels on the same basic level. For various higher levels of higher frequency gives a higher energy difference and thus higher energy photons, ie.
Discrete energy states
The existence of the excited stationary states of the Bohr model of the atom was 1913/1914 detected with the Franck -Hertz experiment. In the experiment, it was shown to mercury atoms in the ground state, that upon impact by a free electron, a certain amount of energy must be transmitted in order to reach the first excited state. Thus the first postulate of the Bohr model of the atom was confirmed in an independent manner.
Weaknesses and contradictions
Some weaknesses and contradictions of the model were clearly already in publication in 1913. Others were later evident with improved experiments and more elaborate theory of quantum mechanics.
- The postulates are justified by any fundamental principle, but solely by their success. They contradict the classical electrodynamics.
- Bohr model describes the behavior of the hydrogen atoms and ions, with only one electron. Many-electron systems are not captured.
- The theory of relativity is considered, even though the electron in the hydrogen ground state almost 1% of the speed of light is attributed.
- The hydrogen atom in Bohr's model would have to be a flat disk.
- Chemical bonds can not be understood with Bohr's model.
- In all stationary states of the web - angular momentum of the electron comes out to large. In particular, he should be in the ground state according to Bohr, but in fact it is 0
- The even-numbered splitting of many spectral lines under the influence of magnetic fields ( anomalous Zeeman effect ) can not be explained.
- Certain spectral lines of hydrogen appears that they are more accurate measurements than double lines. This called after its discoverer Lamb shift separation can not explain the Bohr model.
- The important in radio astronomy 21 -cm line of hydrogen can not be derived from the Bohr model.
- The notion of a defined orbit of the electron around the nucleus violates the uncertainty principle discovered in 1927 by Werner Heisenberg.
The quantum physics, whose statements to date agree in all details with the experimental findings, draws with the orbital model, a fundamentally different picture of the atom. Unlike it accepts the Bohr model, the electrons in the atom everywhere finite probability, even up into the core. They do not move on tracks. Reasonable is the idea of a cloud.
Mathematical formulation
So much goes by the Bohr model of the atom and on the reality, it's far superior to the previous atom models. It allows the comparison of a series of numerical results with experimental results, especially the position of the lines of the hydrogen spectrum. Unlike more modern nuclear models, the mathematics needed for this comes from the insertion into formulas and simple transformations of equations.
Stationary States
The Bohr model treats the electron as a point-like particle that is attracted by the opposite electric charge of the nucleus. This force deflects the path of the electron according to the laws of classical mechanics in circular orbits. That is why it is called the Bohr model the distance of an electron to the core and classic atomic radius. The angular momentum of a particle with mass and velocity on a circular path with the radius is:
On the particle acts a centripetal force
Represented by the electric field of the proton by the Coulomb force
Is given by the dielectric constant.
It follows, first, that the kinetic energy is exactly half as large as the absolute value of the (negative ) potential energy ( when determining the zero point at infinity ):
The angular momentum must satisfy the postulated selection condition:
With the principal quantum number n
By solving for one obtains
And by substituting for the velocity:
Atomic size
Therefore applies to the radius of the orbit:
The smallest radius is called the Bohr atomic radius:
Position of the lines in the spectrum of hydrogen
For the potential energy of the electron in the Coulomb field of the proton is obtained for the -th state
With Rydberg energy (see below). For the kinetic energy ( ibid )
So the total energy
For a nucleus with protons, the energy must be multiplied by:
For the energy difference from the th in the -th state is obtained
Said energy difference is positive, that is, the total energy of the system by external energy supply is increased if, and otherwise energy is emitted. The constant
Is also called the Rydberg energy and widely used in atomic physics as a unit of energy.
For the explanation of the spectra we are interested in the frequency of the emitted light, for after the second Bohr postulate
Applies. The frequency of the emitted radiation when jumping from th -th state in the () is thus
If you press in this formula each frequency according to the wavelength of the corresponding photon, from, arises the Rydberg formula that Johannes Rydberg had already read in 1888 without knowing a model of the atom from the observed line spectra. She agrees in the first four decimal places with the observed values match.
More accurate values are obtained if one considers that the core minimal moves during the electron circles - both move around the common center of gravity, which is very close to the 1836 times heavier proton - the mechanics provides a factor.
If you let go to infinity, we obtain the energy to the state is free for the capture of an electron from infinity, ie, the negative of the total energy of the final state or its ionization energy.
View
The Bohr model of the atom was different extensions in bohr - sommerfeld Atomic Energy Model. As a second and third quantum number has been added to explain intensities and fine structure of the spectral splitting among others. The Stern - Gerlach experiment extended the model again to the spin.
With quantum mechanics, both models were replaced, but at the same time fully justified the Bohr postulates. It was unclear why the Bohr model and its extension in many areas had success, that is correct predictions met.
Swell
- Niels Bohr: On the Constitution of Atoms and Molecules, Part I. In: Philosophical Magazine. 26, 1913, pp. 1-25.
- Niels Bohr: On the Constitution of Atoms and Molecules, Part II Systems Containing Only a Single Nucleus. In: Philosophical Magazine. 26, 1913, pp. 476-502.
- Niels Bohr: On the Constitution of Atoms and Molecules, Part III Systems Containing several nuclei. In: Philosophical Magazine. 26, 1913, pp. 857-875.
- Niels Bohr: The spectra of helium and hydrogen. In: Nature. 92, 1914, pp. 231-232. doi: 10.1038/092231d0.
- Niels Bohr: Atomic Structure. In: Nature. 107, 1921, pp. 104-107. doi: 10.1038/107104a0.
- Michael Eckert: The Birth of the Modern Atomic Theory. In: Physics in our time. 44 ( 4), 2013, pp. 168