Chemokine
Chemokines (name derived from chemotactic cytokines ), a group of cytokines, ie small signal proteins involved in cell migration movement (chemotaxis ) are triggered. The cells thereby move along a concentration gradient to the place of highest chemokine concentration. Chemokines play a central role in the migration of immune cells in the tissue and their emigration from the blood. Some chemokines have an additional activating effect on immune cells, and some are involved in organ development and angiogenesis. Approximately 50 different chemokines are produced by immune cells and many tissue cells. Their effect they exhibit upon binding to chemokine receptors, which are widely used in the immune system. Without the cell migration induced by chemokines, the immune system might not work.
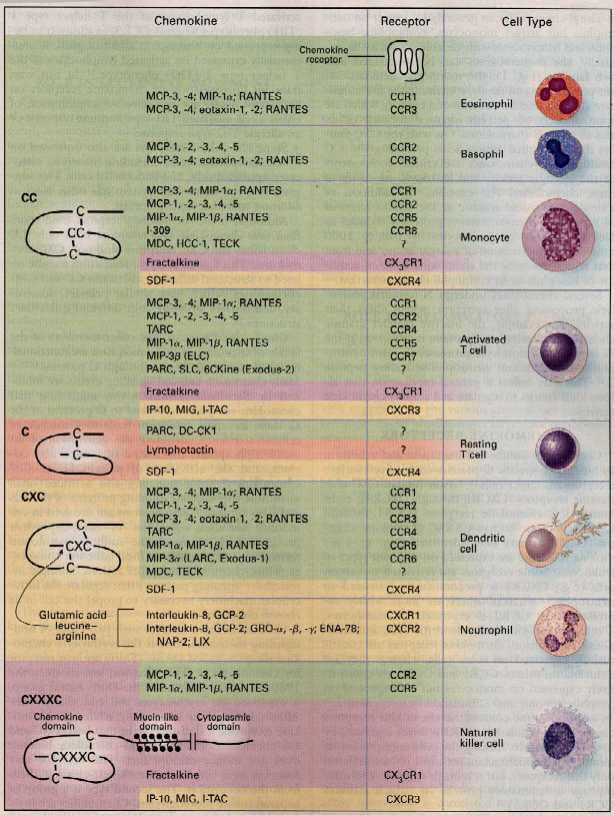
Structure and Nomenclature
Chemokines are small proteins that are composed of approximately 75 to 125 amino acids and having a molecular weight of 8-14 kDa. The amino acid sequence is conserved within the different chemokine family, the homology can be less than 20% or even more than 90%. The spatial convolution or tertiary structure, however, is highly conserved: the amino -terminus is disordered, the main body is formed by a three-stranded antiparallel β -sheet, and the carboxy -terminus ends with an α - helix. Another conserved feature of the chemokines, a group of cysteine residues which can form one or two disulfide bridges.
From the number and position of cysteine residues at the amino terminus, the systematic nomenclature of chemokines derived. Four subfamilies can thus be distinguished: In the CC - chemokines the first two cysteine residues directly follow each other, wherein they are the CXC chemokines are separated by one amino acid, and the CX3C chemokines by three amino acids. These chemokine always two disulfide bonds are formed. In contrast, when there is only one family, the C- conserved cysteine at the amino terminus, and it is only a disulfide bond is formed. The systematic name of the individual chemokines are composed of the name of the family (CC, CXC, CX3C, XC ), the letter L for ligand and a consecutive numbering. In addition, however, the terms are still used frequently, which was given to them in the first description.
Function
The main function of chemokines is the triggering of chemotaxis in immune cells. We can distinguish inflammatory ( or inducible ) and homeostatic (or constitutive ) chemokines. Most chemokines are inflammatory cytokines: Your production is eg triggered by an injury, infection or inflammation, and its release attracts immune cells. Thus, they act as an alarm signal. The homeostatic chemokines on the contrary are constantly being produced and are involved in the organization of lymphoid organs and the monitoring of healthy tissue. This group includes CCL18, CCL19, CCL21, CXCL12, CXCL13 and CXCL14. Another group of chemokines can not be clearly assigned to one of two categories; these are CCL1, CCL17, CCL20, CCL22, CCL25, CXCL9, CXCL10, CXCL11 and CXCL16.
The function of chemokines is not necessarily linked to forms that float freely soluble in the tissue fluid or blood. Concentration gradient can be formed by a tight binding to tissue structures. Chemokines possess many basic amino acids and are therefore positively charged; This enables a strong bonding to the negatively charged sugar molecules ( GAGs ), which are widely distributed on the surface of cells and proteins of the extracellular matrix. Some chemokines, such as CCL2, CCL3 and CCL5 lose their function in the body, when binding to glycosaminoglycans is no longer possible. The exact relationship between binding to tissue structures and function is not yet understood.
Families
CC family
In humans, this family has 24 members, their effect they develop preferentially on neutrophils and T cells.
CXC family
The 16 known human CXC chemokines can be classified by the absence or presence of other structural features, such as the ELR motif consisting of three amino acids. ELR - positive CXC chemokines are considered to be potent promoters of angiogenesis, whereas ELR -negative CXC chemokines generally have an antiangiogenic effect. Receptors for these chemokines are often found on monocytes, eosinophils, basophils, and T- cells.
CX3C family
The 1997 cloned chemokine " fractalkine " is the only member of this family. Characteristic of fractalkine is a CX3C motif and expression of the chemokine domain of a mucinähnlichen, membrane-anchored protein strand. The gene which codes for the CX3C chemokine is located on chromosome 16. Fractalkine is expressed on activated endothelial membrane-bound and can be secreted into the environment. So you exist as membrane-bound or soluble molecules. Fractalkine act on T lymphocytes and monocytes. Soluble fractalkine regulate leukocyte migration. The membrane-bound form provides enhanced adherence of the T- cells and monocytes. Fractalkine are thus able to act directly on the step of the leukocyte extravasation, wherein the cell is converted from rolling on the endothelium in a firm adhesion, followed by the subsequent Leukodiapedese into the tissue. In addition, the expression of fractalkine has been observed in the brain. It is found on activated microglial cells, which suggests that it plays a role in inflammatory processes in the central nervous system. In addition, the chemotaxis and activation of neutrophils is affected in the brains of Fraktalkinen.
C family
XCL1 ( Lymphotactin also or ATAC ) is released primarily by activated CD8 T cells and NK cells. In humans, there is a second gene ( XCL2 ), which is identical to XCL1 up to two amino acids.