Molar concentration
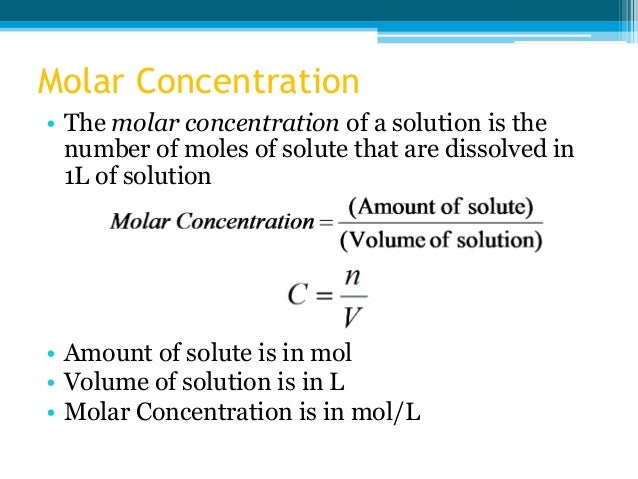
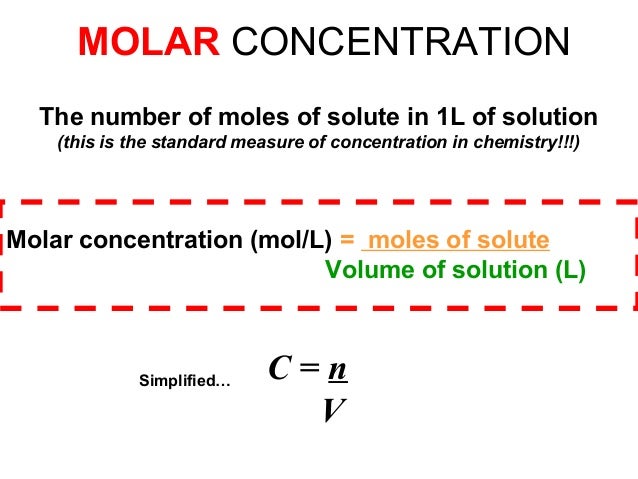
The amount of substance concentration (symbol: c ) or molarity ( obsolete name) is the quotient of the amount of substance (s) of a solute X and the volume (V ) of the solution. With knowledge of the molar mass can be calculated from the amount of substance concentration, mass concentration or - using the Avogadro constant ( see also Avogadro number) - calculate the particle number density.
History
Previously, the symbol M for the unit mole per liter was used and also used together with SI intentions; However, this notation is not with the International System of Units ( SI) compatible. According to IUPAC recommendation only the amount of substance concentration is to be known in English with concentration, but not other concentration variables.
Spelling
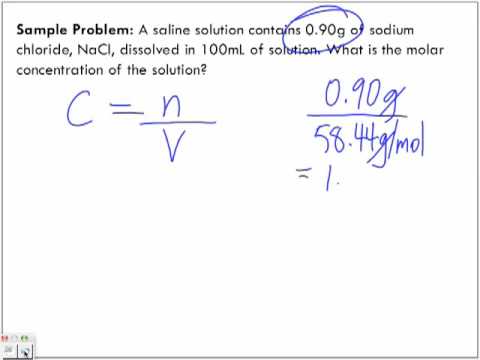
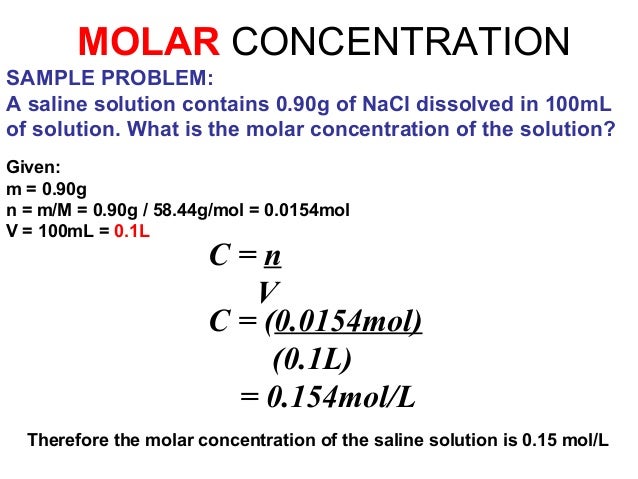
The molar concentration of a substance can be specified in units of moles by liters:
With a 2.5 molar ( outdated representation manner: 2.5 M) sulfuric acid aqueous solution is meant a solution containing 2.5 mol of sulfuric acid per liter of molecules at a given temperature; Here, the common unit mole / l is used.
Worked examples
In a 0.8 - molar ( 0.8 M) solution of sulfuric acid ( molar mass of sulfuric acid: 98.08 g / mol ) are contained per liter of solution 0.8 mol H2SO4. The corresponding mass of H2SO4 is for a volume V = 3 liters of such a sulfuric acid:
The number of particles N a 3 mM ( 3 mM ) solution of sucrose in water at a solution volume V = 2 cubic centimeters, where NA ( Avogadro's number ) is the number of particles per mole is:
Related sizes
- Equivalent concentration
- Content specification, volume concentration
- Molality
- Mass concentration
- Amount of substance
- Osmolarity, osmolality