Auger electron spectroscopy
The Auger electron spectroscopy [ oʒe - ] (AES, after Pierre Auger ) is a spectroscopic method for the highly sensitive and non-destructive study of the chemical composition of a material surface. It is based on the Auger effect, by an atom which is excited in a suitable manner, the electron having a specific, different, depending on the chemical element emits kinetic energy.
Auger effect
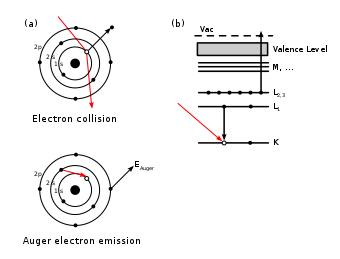
The Auger effect, named after Pierre Auger, is a so-called non-radiative transition in the electron shell of an excited atom. Requirement is that within an atom in an inner electron shell an unoccupied electronic state is present (hole). If it is occupied by an electron from an outer shell again, the energy released can be transferred to another electron of the same atom, so that it leaves the atom as an Auger electron. This effect had already been described Lise Meitner four years before Auger, but their work was little attention. Researchers have identified as both the effect independently of the effect in some recent publications is also called Auger effect Meitner.
To initiate the Auger effect, the atom must first lose one of his tightly bound electrons, which is done by bombarding it with photons or electrons of sufficient energy ( ionization of an inner shell ). If your space is occupied by an electron from an outer shell of the atom again, the Auger effect which thereby released energy is transferred to another electron, which was only weakly bound in the atom, and now flies with a certain kinetic energy which. Auger effect is possible only when the atom on the electron has with suitable energy. He is competing in a radiative transition in which no electron is emitted, but a photon of characteristic X -rays are generated. For the lighter elements clearly outweighs the Auger effect.
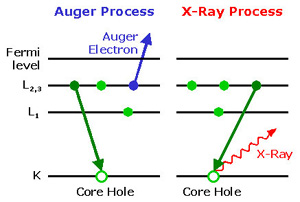
The energy of the Auger electron is determined by the energy levels of the original atom and the remaining ion. It is approximated by assuming that the individual electrons in the atom or ion can occupy well-defined energy levels, all of which are determined by the nuclear charge, ie the chemical atomic number of the atom. Then one has to consider three such levels: the energy of the original hole state, the energy of the energy level, from which changes an electron into the hole, and the energy of the level, from which the finally emitted Auger electron. The possible transitions are therefore named after the three participating electron levels. For example, if a hole in the K shell before, which is filled by an electron from the L-shell, one electron of the M-shell is broadcast (see figure), this transition is called the KLM Auger process. For a more detailed description can be further taken into account that the energy levels shift slightly when their occupation changed with electrons. From the kinetic energy of the emitted electron is so clearly specify to which element the atom belongs. However, the chemical analysis is effectively limited by Auger electron spectroscopy to lighter elements, because with increasing atomic number of the Auger effect strongly decreases in favor of the radiative transition.
Due to conservation of energy, the Auger electron has exactly the same energy as if it were knocked out by a photoelectric effect, caused by a photon of characteristic X-rays, which had been created in the same atom when filling the original hole. For the operation of the process but this is an unacceptable idea, because the Auger effect no photon is generated. Rather, the Auger effect as an elastic collision of two bound electrons in an atom, after the one located in the previously unoccupied hole state and the other in the state of a free particle. Auger effect and photon generation compete with each other, in light atoms the Auger effect usually has the much higher transition probability and therefore anticipates the radiative transition. The Auger effect may also violate applicable in the generation of a photon selection rules.
A special case of the Auger process represents the extremely fast Coster -Kronig transition, for example L1L2M. Here, the original hole L1 from a higher subshell L2 of the same main shell L is filled. If the Auger electron emitted thereby also comes from the same bowl, then one speaks of the super- Coster -Kronig transition, for example L1L2L3. The Coster -Kronig process was named after the two physicists Dirk Coster and Kronig Ralph.
Auger electron spectroscopy
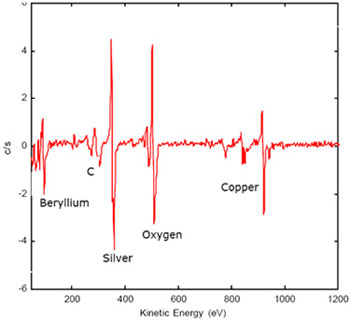
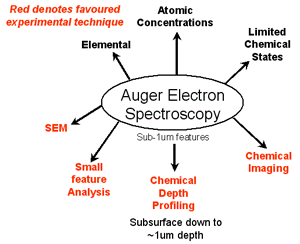
The Auger electron spectroscopy is a very surface-specific method due to the short range of electrons in the relevant energy range (50 eV to 3 keV). The recorded material layer typically includes only the top ten atomic layers. The method can therefore be used for high spatial resolution ( 0.01 microns to 100 microns ) detecting contaminants very efficient. If, by contrast really the pure material are detected and not unintentionally applied impurities that have arisen during sample preparation, they must be removed, for example by sputtering with argon.
A Augerelektronenspektroskop pictures of the type of a scanning electron microscope ( SEM) can be produced. For this, a secondary electron detector is required which converts the secondary electron in a SEM image. Thus, for a similar resolution as an " ordinary " REM achieve. In addition to this function, you can still also use the AES detector for imaging. So can record images that carry material information. This process is called scanning Auger electron microscopy ( engl. scanning Auger microscopy, SAM).
The detection limit with this method is about 0.01-0.1 at%. Only from this value can evaluate the AES peak.
Auger electrons occur even in the photo- electron spectroscopy. The problems caused by the Auger effect peaks are different from the " photopeak " that their energy does not depend on that of the incident ultraviolet or X-ray light.
Auger neutralization
A special case of the general Auger process is in the low energy ion scattering matter. This does not find the Auger transition occurs within an atom. Instead, an unoccupied atomic level of the projectile is filled by an electron from the conduction band of the sample and emits another electron from the conduction band.