Chlorophyll
Chlorophyll ( from Ancient Greek χλωρός chloros " bright green, fresh " and φύλλον phyllon "leaf" ) or leaf green refers to a class of natural dyes, which are formed by organisms engaged in photosynthesis. In particular, plants get their green color by chlorophyll molecules.
Plants, algae and cyanobacteria possess different types of chlorophyll, photosynthesis bacteria driving different types of bacteriochlorophyll.
Structure and Properties
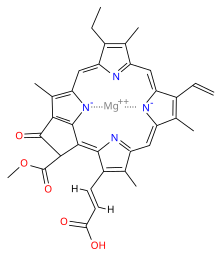
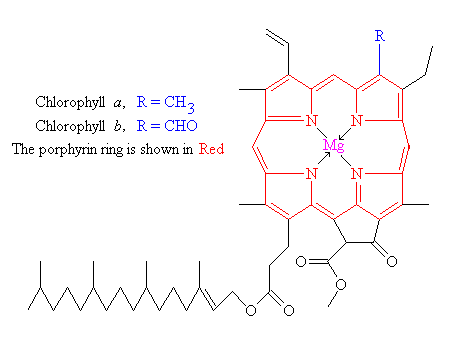
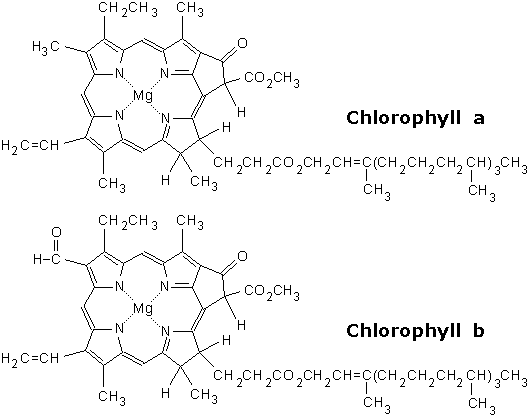
Chlorophylls are chelate complexes consisting of a derivatized porphyrin ring and Mg2 as the central ion. In contrast to the porphyrin the backbone of chlorophylls contains a further, fifth ring on ring III ( numbering according to Fischer). Depending on the nature of the chlorophyll different side chains attached to the basic body. For example, chlorophyll a is esterified with phytol ( see table). Chlorophyllides are chlorophylls without side chains.
Structurally, the chlorophylls are related to the hemes, which, however as part of the blood pigment (hemoglobin ), myoglobin and cytochromes occur as central not magnesium but contain iron. Chlorophylls are readily soluble in ethanol and acetone and similar solvents. Does the photosynthesis in living creatures that release oxygen ( oxygenic phototrophic ), one speaks generally of chlorophyll. However, anoxygenic phototrophic not produce oxygen as a reaction product of photosynthesis in these organisms is referred to as the chlorophyll bacteriochlorophyll.
The absolute configuration of chlorophyll was enlightened by Ian Fleming in 1967.
Chemical structure of oxygenic phototrophs in
Chemical structure in anoxygenic phototrophs: bacteriochlorophylls ( Bchl )
A: No double bond between C7 and C8 ( highlighted in color in the picture) b: In Bchl c, d and e is a mixture of isomers, in which the radical R3 or R4 differently substituted.
Spectral Properties
The absorption spectra of chlorophylls dissolved in solvents always have two distinct absorption maxima, one between 600 and 800 nm, which is called the Qy band, and one at 400 nm, the Soret band is called. The figure at right shows this absorption maxima for chlorophyll a and b. In addition, the Qx - band at 580 nm, which is polarized perpendicular to Qy and absorbs very weakly in the rule exists. For chlorophyll a, they can be seen in the figure yet, for chlorophyll b it disappears underground. The bands located between the region is referred to as a green gap.
Based on the spectra in the figure one can easily understand why leaves - they contain chlorophyll a and b - are green. Together Chlorophyll a and b absorb mainly in the blue spectral range ( 400-500 nm ) and in the red spectral range ( 600-700 nm). In the green area, however, no absorption takes place, so that the green light is scattered, which leaves leaves appear green.
The absorption depends on the solvent, and accordingly, the position of the absorption maxima, depending on the nature of the solvent to some vary only a few nanometers. In the natural environment of chlorophylls, so the protein environment, which is different. Here the position of the absorption maxima of depends on two factors: (1 ) Depending on the partial charge of the surrounding amino acids and bending of the side groups of the chlorophyll molecules, the absorption maxima are at very different wavelengths. ( 2) In proteins, chlorophylls are very close, so that they have an interaction with each other (dipole -dipole interaction, at very low distances exchange interaction ). This interaction results in a decrease of the energy level and thus to a red shift of the absorption maxima. This can be seen in the example of the antenna complex LH2 of purple bacteria is particularly impressive. The LH2 complex consists of two circularly arranged groups of Bacteriochlorophyllmolekülen ( see figure at left ). The upper ring ( B850 ) contains 18 BChl a molecules, that are separated by very small distances, ie they are strongly coupled. The lower ring ( B800 ) consists of 9 BChl a molecules that are significantly further apart and are thus coupled much weaker.
Due to the strong coupling, the absorption of BChl a in the B850 ring is shifted to red. The absorption band located at 850 nm weakly coupled BChl a of the B800 ring, however, absorb at 800 nm, ie approximately the same range as dissolved in solvent BChl a molecules. In the absorption spectrum ( right) of the LH2 complex, the absorption bands of the B800 and the B850 BChl a molecules are clearly separated. In addition, bands are shown, derived from carotenoid molecules, these are not shown in the structure.
Types
There are various types of chlorophyll, which differ in the side groups of the porphyrin. They have different absorption spectra and occur in various phototrophic organisms:
Biosynthesis
Chlorophyll is synthesized in the chloroplasts in eukaryotes, in prokaryotes in the cytoplasm. In many phototrophs chlorophyll formation is induced by light and it has no exposure from. Biosynthesis is a series of many steps with a corresponding number of specific enzymes.
The synthesis of this and other tetrapyrroles is a multistep process, which also has several branching points. The biosynthesis starts from L-glutamate and terminates in a Sirohäm, a heme and chlorophyll a branch.
After several steps uroporphyrinogen III is formed from L- glutamate, the first branch point, can be formed from the Sirohäm. Uroporphyrinogen III is then converted in three steps to protoporphyrin IX, which is the second branching point to the hemes. Protoporphyrin IX in the magnesium ion is introduced in an ATP-dependent reaction, which catalyzes a magnesium chelatase ( EC 6.6.1.1 ). The thus-formed Mg - protoporphyrin IX is converted via Mg - protoporphyrin IX monoethyl to Dinvinyl - protochlorophyllide a. Those step catalyzes a cyclase, which introduces the fifth ring in chlorophyll. In plants the enzyme is O2 - dependent, as it is both O2 - dependent and - independent cyclases in prokaryotes.
In the next step of the D- ring of the Protochloropylls is reduced to a divinyl Chlorophylid by an oxidoreductase ( EC 1.3.1.33 ). In angiosperms, this reaction is completely dependent on light. Therefore, seedlings constitute only Chlorophyll when they are exposed to light. Other plants (some gymnosperms ), algae as well as cyanobacteria have both light-dependent and light- independent oxidoreductase. Consequently, these organisms can synthesize chlorophyll in the dark. Divinyl Chlorophylid A is reduced by a reductase monovinyl Chlorophylid a, before the latter is esterified in a final step by Phytolphosphat for chlorophyll a. This terminal step catalyzes a chlorophyll synthase, a prenyl transferase ( EC 2.5.1.62 ).
For chlorophyll a chlorophyll b or can be reversed formed.
Importance in photosynthesis
Chlorophylls have more tasks of photosynthesis. By far the largest proportion is the absorption of light and the transfer of the absorbed energy. For this purpose, the chlorophyll molecules are organized in light harvesting complexes, which are arranged so that on the one hand the highest possible absorbing surface is formed and on the other hand a funnel -energy created which conducts the absorbed energy to the so-called reaction centers. In the reaction center chlorophylls two serve as an acceptor of this energy. You are so special arranged so that their excitation leads to a charge separation, which can be considered a first step of the actual photosynthesis. This chlorophyll pair is referred to in English as the special pair.
At the very different photosynthetic organisms, there are many differences in the structure of the light -harvesting, the reaction center, however, is almost always the same structure. The special pair is always formed in plants, algae and cyanobacteria of chlorophyll a, in bacteria of different Bakteriochlorophyllen.
Occurrence in food
The chlorophyll content is high, especially in green vegetables. The chlorophyll a and b content of vegetables and fruit per 100 g fresh matter is in the following table arranged in order of decreasing chlorophyll a content listed:
History
First descriptions of a " color fabric ", the ('spirits ' ) can be extracted by ethanol and decomposed under the influence of light, can be found in Heinrich Friedrich Link in his book " fundamental doctrines of the anatomy and physiology of plants ", Göttingen, 1807. Similarly, one finds ambiguous evidence that Joseph Louis Proust has described the green dye as " fécule ". Pierre Joseph Pelletier and Joseph Bienaimé Caventou extracted from the substance again and called him chlorophyll. Initial studies on the chemical structure of chlorophyll are those of Richard Willstätter (1913). The chemist Hans Fischer took Willstätter research in the 1930s, again, in 1940, he was able to explain the structure of the molecule. Fischer's research results were confirmed in 1960 by Robert B. Woodward's chlorophyll synthesis.
Others
An important property of the chlorophyll fluorescence chlorophyll. It is used primarily for determining the chlorophyll content and its activity as well as for other scientific analyzes.
Because of its odor- neutralizing effect of chlorophyll is also available in coated tablet form in the pharmacy, as a remedy for mouth and body odor. As a food additive chlorophyll receives the identification number E 140