Protactinium
{ syn. }
{ syn. }
In traces
{ syn. }
{ syn. }
In traces
In traces
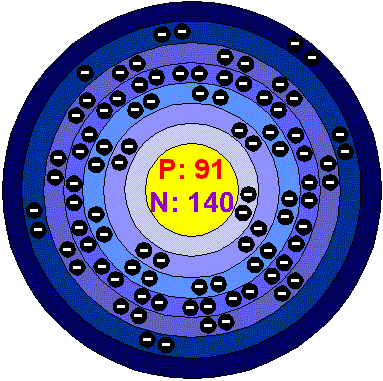
Protactinium is a chemical element with the element symbol Pa and atomic number 91 in the periodic table it is in the group of actinides (7th period, f-block ). It is silvery metallic and becomes superconducting below 1.4 K.
History
234mPa was discovered in 1913 by Kasimir Fajans and Oswald Helmuth Göhring, they gave him because of its short half-life (1.17 minutes) the name Brevium (Latin brevis, short ' ). The durable 231Pa (t ½ = 32,760 years ) was found in 1918 by Otto Hahn and Lise Meitner, they called it Protoactinium ( the chemical element, which is in the decay chain of uranium -235 before the actinium ).
In 1922 Otto Hahn made the further discovery that there are still too the Brevium 234 found by Fajans a second beta radiant isotope with the same mass number 234, which differs from the Brevium only by its longer half-life of 6.7 hours; is this is a rare case of nuclear isomerism.
Aristid of Grosse isolated from the radium waste production in 1927 Protactiniumoxid. Later he also metallic protactinium from Protactiniumiodid dar.
The official name for all three isotopes, as well as all artificially produced isotopes with atomic number 91 was determined in 1949 by IUPAC to protactinium, instead of the more difficult -to-pronounce Protoactinium by Hahn and Meitner.
Occurrence
Protactinium, a radioactive decay product of uranium, found in nature in the form of two isotopes 231Pa and 234Pa, the isotope 234Pa can occur in two different energy states. Protactinium 231Pa, an alpha emitter, is produced from the decay of 235U (see Uranium actinide series ), the beta radiating protactinium 234Pa from the decay of uranium 238U (see uranium-radium series ).
Use
Because of its rarity, high radioactivity and toxicity is protactinium except in research no practical application.
In protactinium 231Pa, which is formed from the decay of uranium 235U and is formed in nuclear reactors by the reaction 232Th n → 231Th 2n and subsequent beta decay, may possibly a nuclear chain reaction take place, which could be used in principle, to build nuclear weapons. The critical mass is stated by Walter Seifritz 750 ± 180 kg. Other authors come to the conclusion that a chain reaction even at arbitrarily large mass in protactinium 231Pa is not possible.
1961 were extracted t of spent nuclear fuel rods in the UK 125 g of protactinium from 60.
Protactinium 233Pa is an intermediate in the breeding process of thorium to uranium 233U 232Th in thorium high- temperature reactors.
Since the availability of modern, highly sensitive mass spectrometer, an application of 231Pa has become possible, for example, as a tracer in Paleoceanography.
Compounds
→ Category: Protactiniumverbindung
Protactinium (V ) chloride ( PaCl5 ) forms yellow monoclinic crystals and has a chain structure consisting of seven -coordinate pentagonal bipyramids.
Safety
Classifications according to the Hazardous Substances Ordinance are not available because they only include the chemical hazard, which plays a very minor role compared to the risks based on the radioactivity in the case of protactinium. Even the latter applies only when there is a relevant matter for that amount.